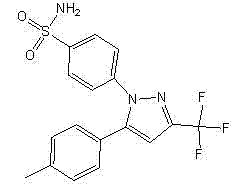
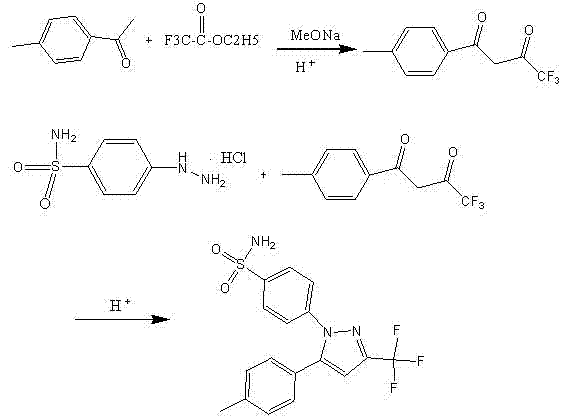
Preparation method of high-yield and high-purity celecoxib
A celecoxib, high-purity technology is applied in the field of preparation of high-yield and high-purity celecoxib, and achieves the effects of simple process, easy large-scale production, and improved yield and purity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Preparation: Add 48.24g of sodium methoxide solution (0.134mol sodium methoxide) to a 250ml three-necked flask, add 19g (0.134mol) of ethyl trifluoroacetate, stir for several minutes, then add 13.5g (0.1mol) of p-methylacetophenone, Heat to 60±3°C for 5 hours to obtain a solution of the intermediate 1-p-methylphenyl-4,4,4-trifluoro-1,3-butanedione, which is set aside.
[0053] Add 23g (0.103mol) of p-hydrazinobenzenesulfonamide hydrochloride, 41.4g of methanol, 27.6g of water, and 11.33g (0.113mol) of hydrochloric acid into a 1000ml three-neck flask, stir, and heat to 60±3°C.
[0054] Add the reaction solution of the obtained intermediate 1-p-methylphenyl-4,4,4-trifluoro-1,3-butanedione dropwise to the solution of p-hydrazinobenzenesulfonamide hydrochloride, and dropwise , 60±3°C for 3 hours, a large amount of off-white solid precipitated. Natural cooling and crystallization for about 12 hours. Filter, wash thoroughly with 50% methanol water, and dry at 60°C for 6 hou...
Embodiment 2
[0057]Preparation: Add 73.3g of sodium isopropoxide solution (0.134mol sodium isopropoxide) into a 250ml three-necked flask, add 19g (0.134mol) of ethyl trifluoroacetate, stir for several minutes, then add 13.5g of p-methylacetophenone ( 0.1mol), heated to 60±3°C and reacted for 5 hours to obtain a solution of the intermediate 1-p-methylphenyl-4,4,4-trifluoro-1,3-butanedione, which was set aside.
[0058] Add 23g (0.103mol) of p-hydrazinobenzenesulfonamide hydrochloride, 41.4g of isopropanol, 27.6g of water, and 11.33g (0.113mol) of hydrochloric acid into a 1000ml three-neck flask, stir, and heat to 60±3°C.
[0059] Add the reaction solution of the obtained intermediate 1-p-methylphenyl-4,4,4-trifluoro-1,3-butanedione dropwise to the solution of p-hydrazinobenzenesulfonamide hydrochloride, and dropwise , 60±3°C for 3 hours, a large amount of off-white solid precipitated. Natural cooling and crystallization for about 12 hours. Filter, wash thoroughly with 50% isopropanol wate...
Embodiment 3
[0062] Preparation: Add 60.76g (0.134mol sodium ethoxide) of sodium ethoxide solution to a 250ml three-necked flask, add 19g (0.134mol) of ethyl trifluoroacetate, stir for several minutes, then add 13.5g (0.1mol) of p-methylacetophenone, Heat to 60±3°C for 5 hours to obtain a solution of the intermediate 1-p-methylphenyl-4,4,4-trifluoro-1,3-butanedione, which is set aside.
[0063] Add 23g (0.103mol) of p-hydrazinobenzenesulfonamide hydrochloride, 41.4g of ethanol, 27.6g of water, and 11.33g (0.113mol) of hydrochloric acid into a 1000ml three-neck flask, stir, and heat to 60±3°C.
[0064] Add the reaction solution of the obtained intermediate 1-p-methylphenyl-4,4,4-trifluoro-1,3-butanedione dropwise to the solution of p-hydrazinobenzenesulfonamide hydrochloride, and dropwise , 60±3°C for 3 hours, a large amount of off-white solid precipitated. Natural cooling and crystallization for about 12 hours. Filter, wash thoroughly with 50% ethanol water, and dry at 60°C for 6 hours t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com