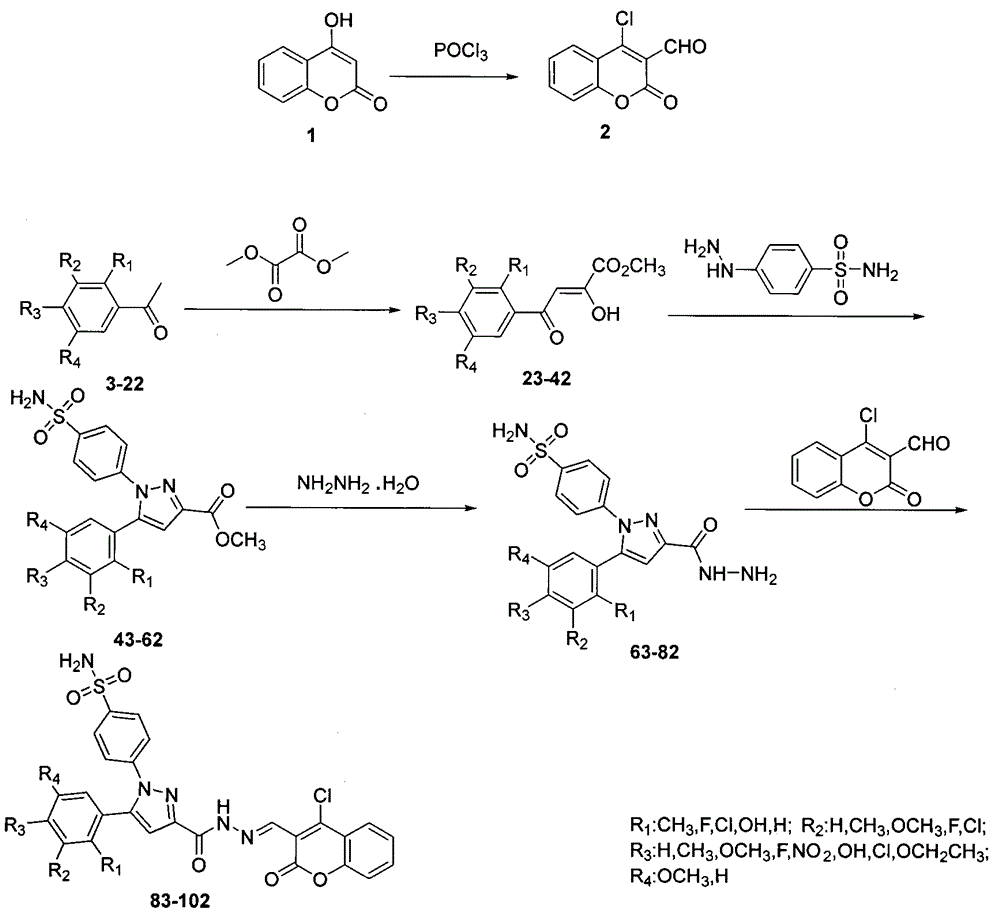
Preparing method for 4-(3-(3-(4-clocoumarol)-acylhydrazone)-5-phenyl-pyrazol) benzene sulfonamide derivate and application to anti-cancer drugs
A kind of technology of chlorocoumarin and benzenesulfonamide, applied in 4-(3-(3-(4-chlorocoumarin)-acylhydrazone)-5-phenyl-pyrazole)benzenesulfonamide derivatives The preparation and application of anticancer drugs can solve the problems of adverse reactions, reduced scope of use, and easy generation of drug resistance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
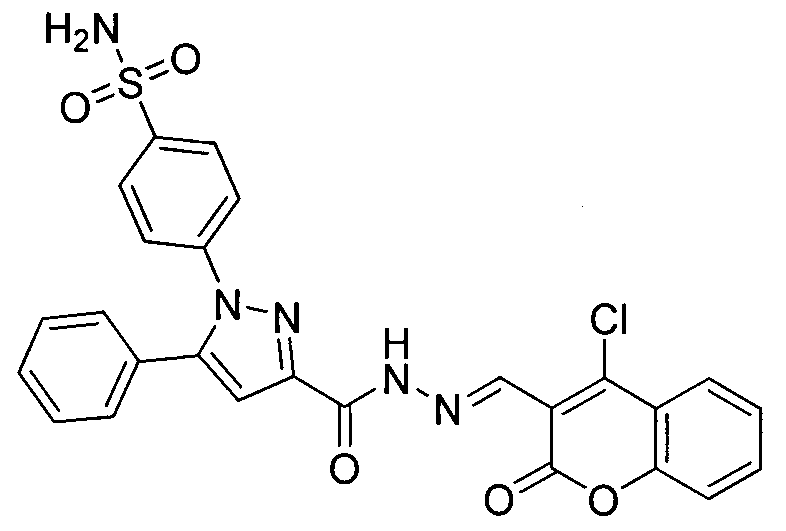
[0016] Example 1: Preparation of 4-(3-(3-(4-chlorocoumarin)-acylhydrazone)-5-phenyl-pyrazole)benzenesulfonamide
[0017]
[0018] Under stirring, 5-phenylpyrazole hydrazide benzenesulfonamide (0.27g, 0.5mmol), ethanol (15mL), 4-chloro-1-benzopyran-2-one (0.12g, 0.6mmol), Acetic acid (0.5mL) was added in a 50mL round-bottomed flask; the reaction was stirred at room temperature for 12h, followed by TLC (developer V AcOEt :V 正己烷 = 1:2), after the reaction, the reaction mixture was added to ice water, filtered, the solid was washed with distilled water, and finally vacuum-dried, and the obtained solid was dissolved in absolute ethanol for recrystallization and purification to obtain the powdery target compound.
[0019] Yellow solid, yield 80.4%, m.p.243~248℃; 1 H NMR (DMSO-d 6 , 300MHz) δ: 12.25 (s, 1H, CONH), 8.74 (s, 1H, CHN), 8.04 (d, J=6.0Hz, 1H, ArH), 7.90 (d, J=6.4Hz, 2H, ArH) , 7.79~7.75(m, 1H, ArH), 7.61(d, J=6.4Hz, 2H, ArH), 7.53(t, J=7.5Hz, 4H, ArH and SO 2 NH ...
Embodiment 2
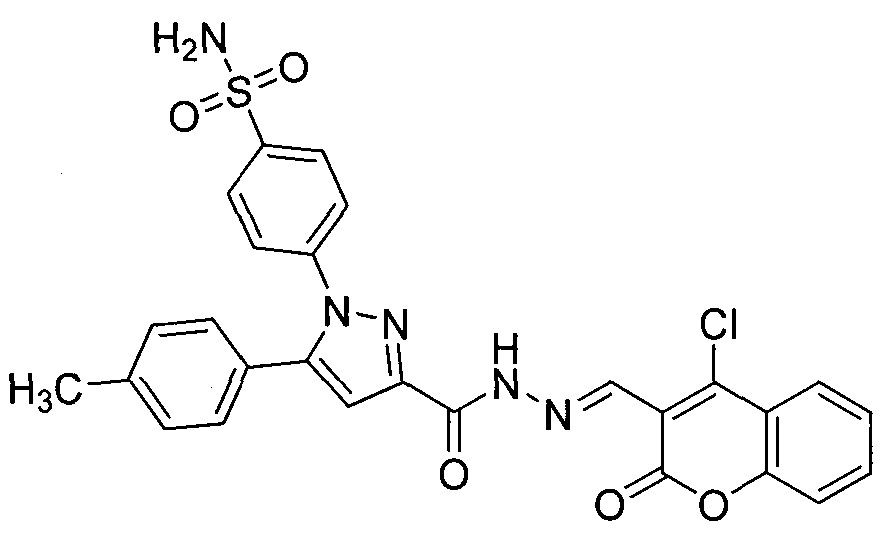
[0020] Example 2: Preparation of 4-(3-(3-(4-chlorocoumarin)-acylhydrazone)-5-(4-methylphenyl)-pyrazole)benzenesulfonamide
[0021]
[0022] The preparation method is the same as in Example 1. A brown solid was obtained with a yield of 66.8%. m.p.238~241°C; 1 H NMR (DMSO-d 6 , 300MHz) δ: 12.23 (s, 1H, CONH), 8.73 (s, 1H, CHN), 8.03 (d, J=6.0Hz, 1H, ArH), 7.89 (d, J=6.4Hz, 2H, ArH) , 7.78~7.74(m, 1H, ArH), 7.60(d, J=6.4Hz, 2H, ArH), 7.52(d, J=6.0Hz, 4H, ArH and SO 2 NH 2 ), 7.23(s, 4H, ArH), 7.17(s, 1H, CH), 2.32(s, 3H, CH 3 ).ESI-MS: 563.1[M+H] + .Anal.Calcd for C 27 h 20 ClN 5 o 5 S: C, H, N.
Embodiment 3
[0023] Example 3: Preparation of 4-(3-(3-(4-chlorocoumarin)-acylhydrazone)-5-(3-methylphenyl)-pyrazole)benzenesulfonamide
[0024]
[0025] The preparation method is the same as in Example 1. A yellow solid was obtained with a yield of 76.6%. m.p.214~217°C; 1 H NMR (DMSO-d 6 , 300MHz) δ: 12.23 (s, 1H, CONH), 8.74 (s, 1H, CHN), 8.04 (d, J=6.0Hz, 1H, ArH), 7.90 (d, J=6.4Hz, 2H, ArH) , 7.77(t, J=6.0Hz, 1H, ArH), 7.61(d, J=6.4 Hz, 2H, ArH), 7.51(d, J=6.0Hz, 4H, ArH and SO 2 NH 2 ), 7.25(t, J=6.0Hz, 3H, ArH), 7.20(s, 1H, CH), 7.03(d, J=6.0Hz, 1H, ArH), 2.30(s, 3H, CH 3 ).ESI-MS: 563.1[M+H] + .Anal.Calcd for C 27 h 20 ClN 5 o 5 S: C, H, N.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com