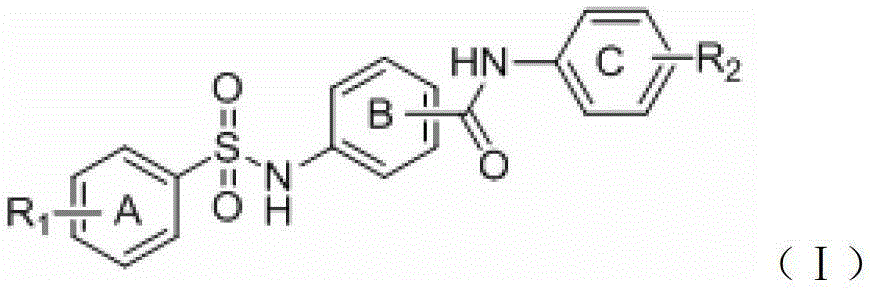
A group of benzene sulfonamido benzamide derivatives, and preparation and application thereof
A kind of technology of benzenesulfonamidobenzamide and derivatives, applied in the field of pharmacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
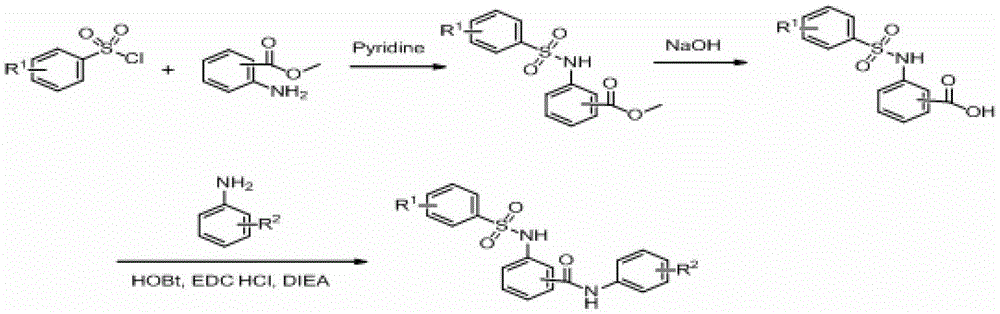
Embodiment 1
[0023] (a) Preparation of 2-(3-nitrobenzenesulfonamido)methyl benzoate
[0024] Add methyl anthranilate (10g, 42.8mmol) and pyridine (3.4mL, 42.8mmol) into tetrahydrofuran (120mL), stir and react at 25°C for 0.5 hours, then add dropwise 3-nitrobenzenesulfonyl chloride (5.4mL , 42.8mmol), and the reaction solution was reacted at 25°C for 9 hours. The reaction liquid was evaporated under reduced pressure to remove tetrahydrofuran, and the residual liquid was poured into 100 mL of water, and a large amount of red solid was precipitated, and 5% hydrochloric acid was added dropwise until the pH value of the solution was 5, and after stirring for 0.5 hours, suction filtered, and the filter cake was washed with water to the filtrate It is neutral, and the filter cake is vacuum-dried to obtain 10.6 g of red powder solid, with a yield of 74%.
[0025] 1 H-NMR(400MHz,DMSO):3.75(3H,s),7.25(1H,t,J=6.0Hz),7.39(1H,d,J=6.0Hz),7.57(1H,t,J=6.0Hz ),7.79(1H,dd,J 1 =6.0Hz,J 2 =1.2Hz),7.85(1H...
Embodiment 2
[0033] (a) Preparation of N-(3-hydroxy-4-methoxyphenyl)-2-(3-nitrobenzenesulfonamido)benzamide
[0034] Prepared according to the method of Example 1 step (c), using the product of Example 1 step (b) and 3-hydroxyl-4-methoxyaniline to prepare N-(3-hydroxyl-4-methoxyphenyl )-2-(3-nitrobenzenesulfonamido)benzamide compound, in the form of off-white powder, with a yield of 65.1%.
[0035] 1 H-NMR(400MHz,DMSO):3.30(3H,s),7.01(1H,d,J=6.0Hz),7.23(1H,t,J=6.0Hz),7.35-7.40(3H,m),7.44 -7.47(1H,m),7.49-7.52(2H,m),8.03(1H,t,J=6.4Hz),8.45(1H,d,J=6.4Hz),8.67(1H,d,J=6.4Hz ),8.73(1H,s),10.24(1H,s).MS m / z:442.08[M-H] - .
Embodiment 3
[0037] (a) Preparation of N-(3,5-dichlorophenyl)-2-(3-nitrobenzenesulfonamido)benzamide
[0038] Prepared according to the method of step (c) of Example 1, using the product of step (b) of Example 1 and 3,5-dichloroaniline to prepare N-(3,5-dichlorophenyl)-2-(3 -Nitrobenzenesulfonamido) benzamide compound, in the form of off-white powder, with a yield of 72.3%.
[0039] 1H-NMR(400MHz,DMSO):7.30-7.36(3H,m),7.51(1H,t,J=8.0Hz),7.60(1H,d,J=7.2Hz),7.64(2H,d,J= 2.0Hz), 7.73(1H,t,J=8.0Hz),8.06(1H,d,J=8.4Hz),8.31-8.33(1H,m),8.40-8.41(1H,m),10.31(1H, s),10.45(1H,s).MS m / z:464.07[M-H] - .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com