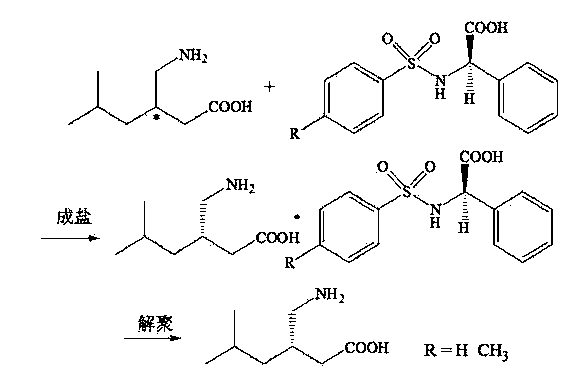
Chemical resolution preparation method of S-configuration pregabalin
A pregabalin and chemical separation technology, applied in the S-configuration pregabalin separation process and compound preparation field, to achieve the effects of less environmental pollution, high optical purity and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] (1) Salt forming process
[0023] In a 50mL clean three-neck flask, add 5.50g of p-toluenesulfonamido-D-phenylglycine and 40mL of isopropanol solution with a volume ratio of 3%, and heat to dissolve; add 2.40g of pregabalin racemate, and keep warm at a slow speed Stir for 0.5h, cool down naturally at 30-35°C, and crystallize for 4h. Suction filtration, transfer the obtained solid to 15 mL of isopropanol solution with a volume ratio of 3%, keep stirring at 50°C for 1h, cool to 30°C, crystallize for 1h, suction filtration, and dry to obtain 3.0g of S-pregabalin and p- Methylbenzenesulfonamide-D-phenylglycinate complex.
[0024] (2), depolymerization process
[0025] Suspend 3.0 g of the above-mentioned salt complex in 15 mL of deionized water, adjust pH = 0.5-1 with 10% hydrochloric acid, stir at room temperature for 3 h, filter, wash with 4 mL of water, dry, and recover the resolving agent p-toluenesulfonamide -D-phenylglycine 2.0 g. Adjust the pH = 6 to 6.5 with 20%...
Embodiment 2
[0027] (1) Salt forming process
[0028] In a 100mL clean three-necked flask, add 5.0g of benzenesulfonamide-D-phenylglycine and 40mL of 3% isopropanol solution by volume, heat to dissolve; add 2.40g of pregabalin racemate, keep warm and stir slowly for 0.5h , natural cooling 30 ~ 35 ℃, crystallization 6h. Suction filtration, the obtained solid was transferred to 30 mL of isopropanol solution with a volume ratio of 3%, kept stirring at 50°C for 2h, cooled to 30°C, crystallized for 3h, suction filtration, and dried to obtain 2.80g of S-pregabalin and benzene Sulfonamide-D-phenylglycinate complex.
[0029] (2), depolymerization process
[0030]Suspend 2.80 g of the above salt complex in 15 mL of deionized water, adjust pH = 0.5-1 with 10% hydrochloric acid, stir at room temperature for 3 h, filter, wash with 5 mL of water, dry, and recover the resolving agent benzenesulfonamide group-D- Phenylglycine 1.8g. Use 10% sodium hydroxide to adjust pH = 6 to 6.5. Concentrate 8 mL o...
Embodiment 3
[0032] (1) Salt forming process
[0033] In a 50mL clean three-neck flask, add 5.50g of p-toluenesulfonamido-D-phenylglycine and 45mL of isopropanol solution with a volume ratio of 3%, and heat to dissolve; add 2.40g of pregabalin racemate, and keep warm at a slow speed Stir for 0.5h, cool down naturally at 30-35°C, and crystallize for 6h. Suction filtration, the obtained solid was transferred to 15 mL of isopropanol solution with a volume ratio of 3%, kept stirring at 50°C for 2h, cooled to 30°C, crystallized for 2h, suction filtration, and drying to obtain 3.10g of S-pregabalin and para Methylbenzenesulfonamide-D-phenylglycinate complex.
[0034] (2), depolymerization process
[0035] Dissolve 3.10 g of the above-mentioned salt complex in 20 mL of 50% methanol solution by volume, adjust the pH = 6.5-7 with ammonia water, stir at room temperature for 3 h, cool down to 0 ° C, keep warm overnight, filter, wash with 2 mL of cold water, and dry to obtain White S-pregabalin 0.7...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com