Bicyclol phosphate ester compound and preparing method, preparation and application thereof
A technology for bicyclic alcohol phosphate esters and compounds, which is applied in the directions of phosphorus organic compounds, chemical instruments and methods, compounds of elements of Group 5/15 of the periodic table, etc. It can improve the route of administration, prolong the retention time, and improve the water solubility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] The preparation of embodiment 1 bicyclic alcohol phosphate
[0086] Dissolve phosphoric acid (56ml, 0.96mol) in acetonitrile (800ml), slowly add pyridine (320ml), triethylamine (224ml), acetic anhydride (152ml), bicyclic alcohol (80g, 0.205mol) under stirring in a water bath at room temperature, and reflux Under the conditions, react until the raw materials disappear (about 20 hours), let stand at room temperature for 6-8 hours, add water (100ml) to the reaction solution, and continue to reflux for 2h, then concentrate under reduced pressure to remove the solvent, add water and ethyl acetate to the concentrate for extraction, water Layer through LSA-21 macroporous resin (1kg) column, gradient elution with water and ethanol, the product eluate was concentrated and dried to give bicyclyl phosphate (44g, 45.6%).
Embodiment 2
[0087] The preparation of embodiment 2 bicyclol calcium phosphate
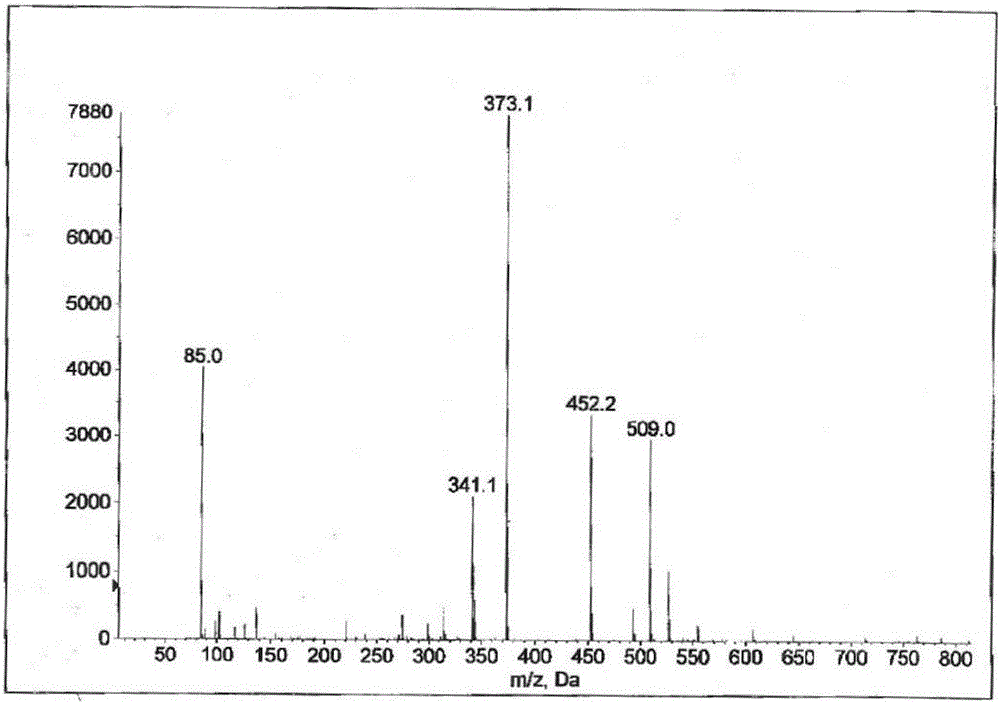
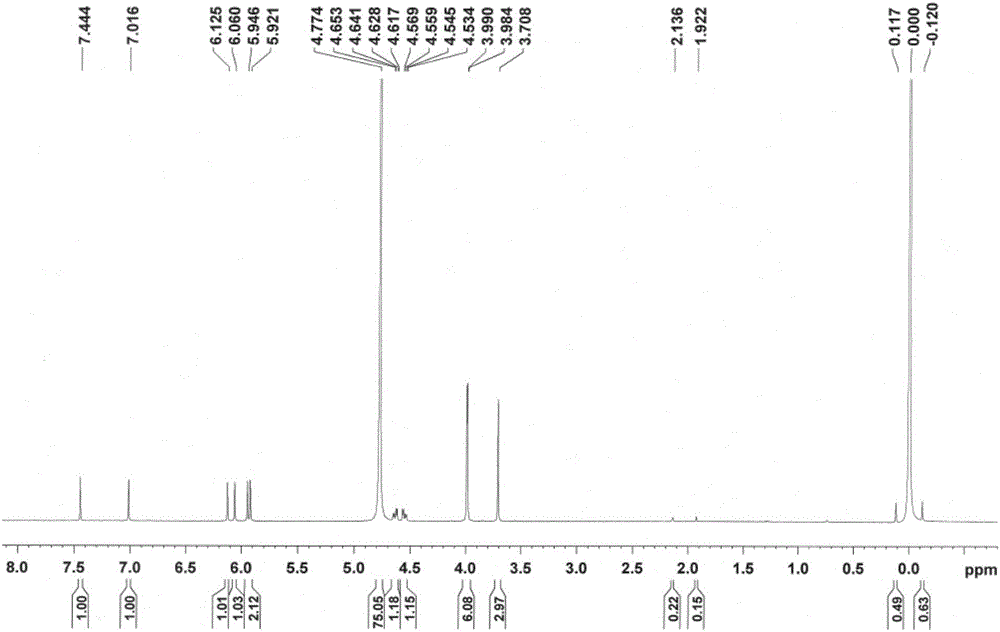
[0088] Dissolve bicyclyl phosphate (16g, 0.034mol) in 500ml of methanol, slowly add calcium acetate (8g, 0.051mol) water (32ml) solution dropwise, stir at room temperature for 30min, filter to obtain a white solid, add this solid to 10% In methanol aqueous solution (2000ml), reflux and stir for 4h, hot filter, the filtrate is extracted three times with ethyl acetate, the water layer is concentrated under reduced pressure to 200ml, stand for crystallization for 2h, filter and dry to get bicycloalcohol calcium phosphate (10g, 57.6% ).
[0089] ESI-MS: m / z[M-H]=509.0
[0090] HNMR (D 2 O, 500MH Z )δ3.708(s, 3H); 3.984, 3.990(d, 6H); 5.921, 5.946, 6.060, 6.125(s, 4H); 4.534-4.653(q, 3H); s, 1H).
Embodiment 3
[0091] The preparation of embodiment 3 bicyclyl sodium phosphate
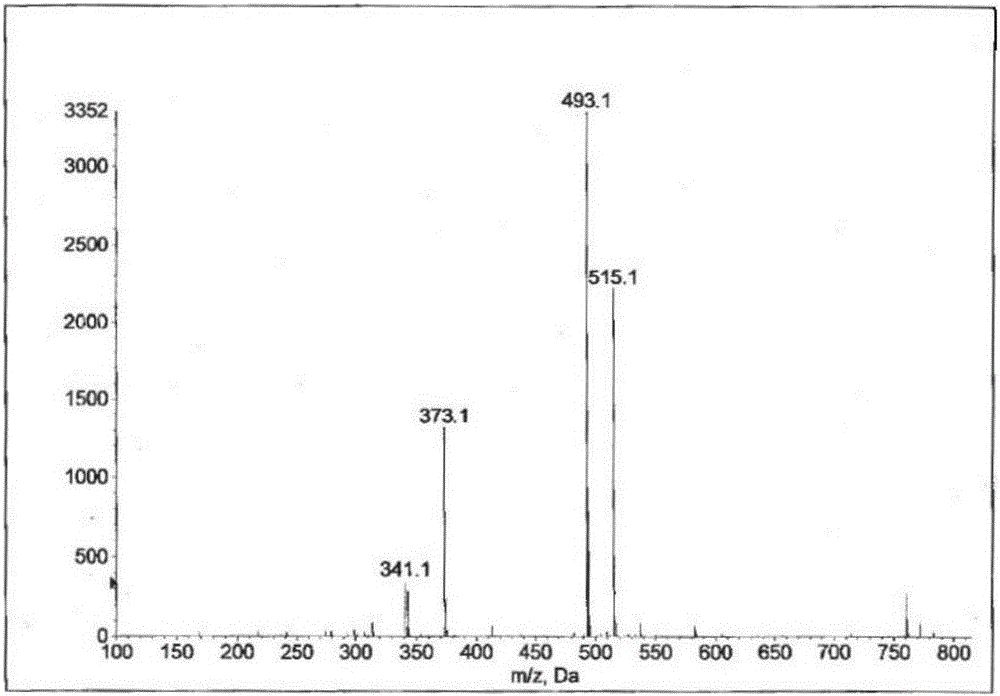
[0092]Take bicyclyl calcium phosphate (3g, 0.006mol), sodium carbonate (0.6g, 0.006mol), water (50ml), stir at room temperature for 2h, filter, and extract the filtrate three times with ethyl acetate, concentrate the water layer to dryness under reduced pressure, add Anhydrous methanol (80ml), stirred in a water bath at 50°C for 30min, filtered out insoluble matter, the filtrate was concentrated to dryness under reduced pressure, added absolute ethanol (50ml)), stirred and dissolved in a water bath at 80°C, frozen and crystallized, filtered and dried to obtain bicyclic alcohol phosphoric acid Sodium (2.0 g, 64.5%).
[0093] ESI-MS: m / z[M-H]=515.1
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com