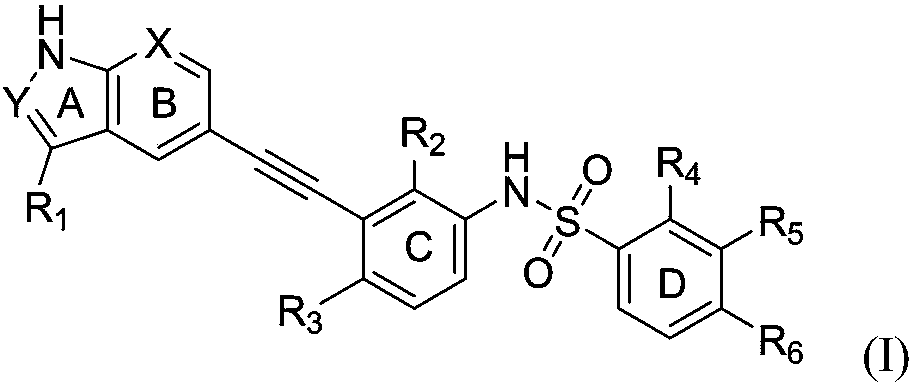
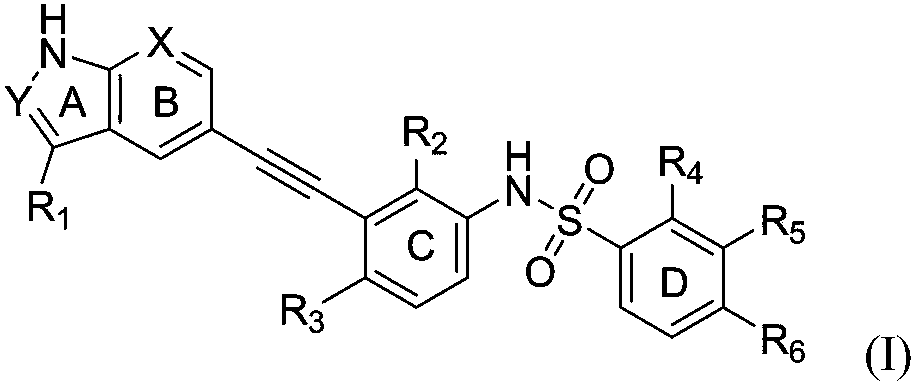
Alkylene phenylsulfonamide type selective ZAK inhibitor and application thereof
A technology of alkynephenylbenzenesulfonamide and benzenesulfonamide, applied in the field of alkynephenylbenzenesulfonamide selective ZAK inhibitors, can solve the problems of limited research and lack of ZAK selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105] Example 1: 3-chloro-N-(2,4-difluoro-3-((3-methoxy-1H-pyrazolo[3,4-b]pyridin-5-yl)ethynyl)benzene base)-4-fluorobenzene (designated as CYD2823)
[0106]
[0107] Step a: Preparation of 5-bromo-1H-pyrazolo[3,4-b]pyridin-3(2H)-one (Compound 1)
[0108]
[0109] 5-Bromo-2-chloronicotinic acid methyl ester (50g, 200mmol) was dissolved in 500mL ethanol, 80% hydrazine hydrate (37.5g, 600mmol) was added with stirring, and heated to reflux for 12h. After cooling to room temperature and adding a large amount of ice water, a light yellow solid precipitated out. It was filtered under reduced pressure, and the filter cake was fully washed with water and dried in vacuo to obtain 36 g of a light yellow solid (Yield: 85%). 1 H NMR (400MHz, DMSO-d 6 ) δ 12.41 (s, 1H), 11.04 (s, 1H), 8.47 (d, J=2.0Hz, 1H), 8.30 (d, J=2.0Hz, 1H). MS(ESI),m / z:214[M+H] + .
[0110] Step b: Preparation of 5-bromo-1-(4-methoxybenzyl)-1H-pyrazolo[3,4-b]pyridin-3(2H)-one (Compound 2)
[0111]
[...
Embodiment 2
[0138] Example 2: 3-chloro-N-(2,4-difluoro-3-((3-methoxy-1H-pyrazolo[3,4-b]pyridin-5-yl)ethynyl)benzene base)-2-fluorobenzene (named as CYD2826) preparation
[0139]
[0140] The synthesis method is as in Example 1.
[0141] 1 H NMR (400MHz, DMSO-d 6 )δ12.95(s,1H),10.84(s,1H),8.61(d,J=2.0Hz,1H),8.33(d,J=1.2Hz,1H),7.95(t,J=7.0Hz, 1H), 7.69 (t, J=6.8Hz, 1H), 7.41-7.31 (m, 2H), 7.25-7.21 (m, 1H), 4.02 (s, 3H).
[0142] 13 C NMR (150MHz, DMSO-d 6 )δ160.23(d, J=252.0Hz, 1C), 156.81(d, J=253.5Hz, 1C), 155.11, 153.77(d, J=255.0Hz, 1C), 152.20, 151.06, 136.16, 132.61, 129.86 (d,J=10.5Hz,1C),129.27(d,J=13.5Hz,1C),128.82,126.03(d,J=4.5Hz,1C),121.68(d,J=16.5Hz,1C),120.35 (d, J=12Hz, 1C), 112.26 (d, J=22.5Hz, 1C), 109.44, 103.20, 101.75 (t, J=19.5Hz, 1C), 97.74, 76.18, 55.99.
[0143] HRMS (ESI) calcd for C 21 h 12 CIF 3 N 4 o 3 S[M+H] + ,493.0344; found, 493.0348.
[0144] HPLC purity=99.32%, Rt 10.82min.
Embodiment 3
[0145] Example 3: N-(2,4-difluoro-3-((3-methoxy-1H-pyrazolo[3,4-b]pyridin-5-yl)ethynyl)phenyl)-2 -Preparation of toluenesulfonamide (designated as CYD2830)
[0146]
[0147] The synthesis method is as in Example 1.
[0148] 1 H NMR (400MHz, DMSO-d 6 )δ12.94(s,1H),10.37(s,1H),8.60(d,J=2.0Hz,1H),8.32(d,J=1.2Hz,1H),7.71(d,J=8Hz,1H ),7.55-7.52(m,1H),7.43(d,J=7.6Hz,1H),7.35-7.26(m,2H),7.19(t,J=8.8Hz,1H),4.02(s,3H) ,2.63(s,3H).
[0149] HRMS (ESI) calcd for C22 h 16 f 2 N 4 o 3 S[M+H] + ,455.0984; found, 455.0980.
[0150] HPLC purity=95.95%, Rt 14.10min.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com