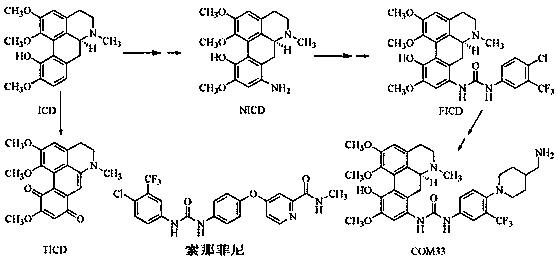
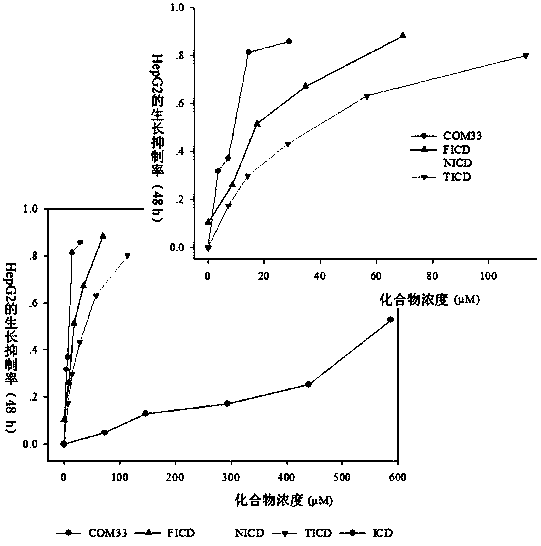
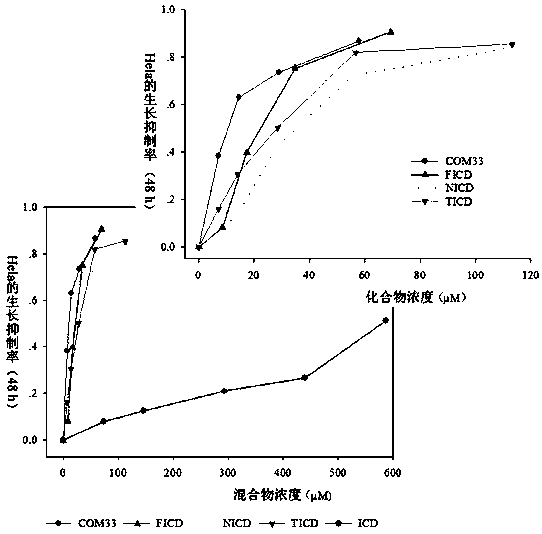
Isocorydine derivative, preparation method and applications thereof
一种异紫堇碱、衍生物的技术,应用在异紫堇碱的化学结构衍生物及其制备领域,能够解决药物毒副作用、耐药性影响患者生活质量和治疗效果等问题,达到好抗癌活性、改善体内药代动力学行为、增强体内药理活性的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1: Synthesis of 8-(N-(3'-trifluoromethyl-4'-chloro-phenyl))-ureido-isocordyrine (FICD).
[0052] The NICD compound (0.7915 g, 2.23 mmol) was dissolved in 50 mL of dichloromethane, and the solution was cooled to below 0 °C in ice-brine. Another 4-chloro-3-trifluoromethylphenyl isocyanate (0.5822 g, 2.63 mmol) was added to 50 mL of dichloromethane to dissolve the compound, cooled to below 0°C, and added dropwise to the above NICD solution under mechanical stirring During the process, the mixture was mechanically stirred for 90 min, and the end of the reaction was detected by TLC, and the reaction was stopped. Use ammonia water to adjust the pH value of the above reaction solution, add 100×3mL dichloromethane respectively, extract three times, combine the organic phases, recover the organic solvent, separate by silica gel column chromatography, petroleum ether: ethyl acetate: methanol = 6:2:1, 0.9278 g of the target compound FICD was obtained with a yield of 72.3%...
Embodiment 2
[0055] Example 2: Synthesis of 8-(N-(3',4'-dichloro-phenyl))-ureido-isocordyline.
[0056] The NICD compound (0.0906 g, 0.25 mmol) was dissolved in 50 mL of dichloromethane, and the solution was cooled to below 0 °C in ice-brine. Another 3,4-dichlorophenyl isocyanate (0.0480 g, 0.25 mmol) was added to 50 mL of dichloromethane to dissolve the compound, cooled to below 0°C, added dropwise to the above NICD solution under mechanical stirring, and mechanically stirred After 90 min, TLC detected the reaction end point, and stopped the reaction. Use ammonia water to adjust the pH value of the above reaction solution, add 100×3 mL dichloromethane respectively, extract three times, combine the organic phases, recover the organic solvent, and separate by silica gel column chromatography, petroleum ether: ethyl acetate: methanol = 6:2:1 , to obtain 0.0825 g of the target compound 8-(N-(3',4'-dichloro-phenyl))-ureido-isocordyrine with a yield of 46.9%. Compounds were detected as target...
Embodiment 3
[0059] Example 3: Synthesis of 8-(N-p-methylphenyl)-ureido-11-O-p-toluyl-isocordyline.
[0060] The NICD compound (0.3032 g, 0.85 mmol) was dissolved in 50 mL of dichloromethane, and the solution was cooled to below 0 °C in ice-brine. Another 4-tolyl isocyanate (0.1137 g, 0.85 mmol) was added to 50 mL of dichloromethane to dissolve the compound, cooled to below 0°C, added dropwise to the above NICD solution under mechanical stirring, mechanically stirred for 90 min, and TLC Detect the end point of the reaction and stop the reaction. Use ammonia water to adjust the pH value of the above reaction solution, add 100×3 mL dichloromethane respectively, extract three times, combine the organic phases, recover the organic solvent, and separate by silica gel column chromatography, petroleum ether: ethyl acetate: methanol = 6:2:1 , 0.1628 g of the target compound 8-(N-p-methylphenyl)-ureido-11-O-p-toluyl-isocordyrine was obtained, with a yield of 30.7%. Compounds were detected as targ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com