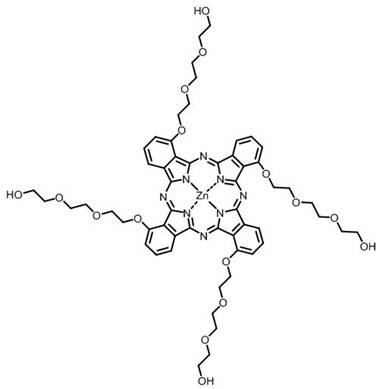
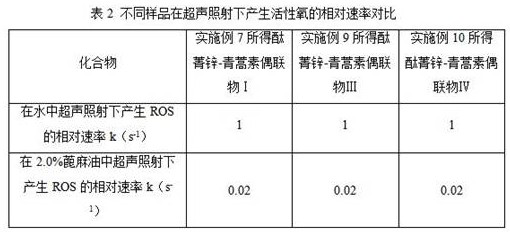
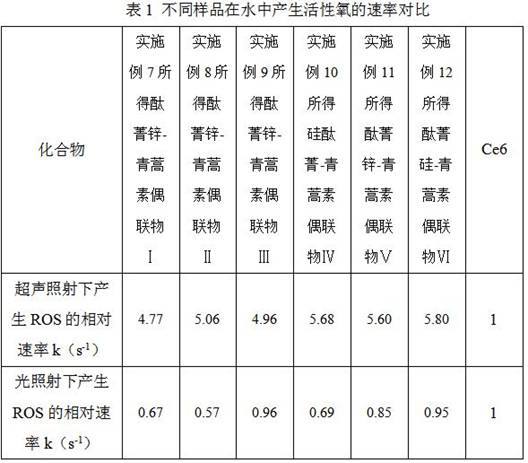
Phthalocyanine-artemisinin conjugate used as sonodynamic/photodynamic sensitizer as well as preparation method and application of phthalocyanine-artemisinin conjugate
A conjugate, artemisinin technology, applied in chemical instruments and methods, compounds of Group 4/14 elements of the periodic table, medical preparations of inactive ingredients, etc. Dynamic therapy needs to be improved to achieve good anti-tumor effect, excellent synergistic anti-tumor, and excellent tumor targeting effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] (1) 3-(2-(2-hydroxyethoxy)ethoxy)ethoxyphthalonitrile ( )Synthesis:
[0043] Add (5~50 mmol, 10 mmol in this example) triethylene glycol and potassium carbonate (10~40 mmol, 20 mmol in this example) into DMF (10~50 mL, 35 mL in this example), Stir for 10 minutes (under the protection of nitrogen, slow down the flow rate and stirring speed), then add 3-nitrophthalonitrile (5 mmol), and stir at room temperature for 12 h under the protection of nitrogen. TLC detection reflected the situation. After the reaction was completed, the reaction solution was concentrated by rotary evaporation, and the concentrated solution was added to ice water to precipitate a light yellow solid. The filter cake was collected by suction filtration with a Buchner funnel and dried in a vacuum oven to obtain a solid product. The yield was 54.35%.
[0044] Characterization data: 1 H NMR (400 MHz, CDCl 3 ) δ 7.66 (t, J = 8.2 Hz, 1H), 7.38 (d, J = 7.7 Hz, 1H), 7.32 (d, J = 8.7 Hz, 1H), 4.41...
Embodiment 2
[0049] (1) 3-(2-hydroxyethoxy)ethylmercaptophthalonitrile ( )Synthesis:
[0050] Add (5~50 mmol, 10 mmol in this example) 2-mercaptoethoxyethanol and potassium carbonate (10~40 mmol, 20 mmol in this example) to DMF (10~50 mL, choose in this example 35mL), stirred for 10 minutes (nitrogen protection, slow flow and stirring speed), then added 3-nitrophthalonitrile (5mmol), stirred at room temperature for 12-24h under nitrogen protection. TLC detection reflected the situation. After the reaction was completed, the reaction solution was concentrated by rotary evaporation, and the concentrated solution was added to ice water to precipitate a yellow solid. The filter cake was collected by suction filtration with a Buchner funnel and dried in a vacuum oven to obtain a solid product. The yield was 86.65%.
[0051] Characterization data: 1 H NMR (400 MHz, DMSO- d 6) δ 7.74 (dd, J = 7.6, 1.3 Hz, 1H),7.65-7.57 (m, 2H), 3.78 (t, J = 6.2 Hz, 2H), 3.73-3.70 (m, 2H), 3.61-3.57 (m,2H)...
Embodiment 3
[0056] (1) 3,6-bis-(2-(2-hydroxyethoxy)ethoxy)ethoxyphthalonitrile ( )Synthesis:
[0057] Measure triethylene glycol (100~300 mmol, choose 180 mmol in this example), triethylamine (2~10 mL, choose 5 mL in this example) 20 mL for the example) and stirred at 0°C for 20 min; weigh p-toluenesulfonyl chloride (30 mmol) plus dichloromethane (5~30 mL, 20 mL for this example) ) dissolved, slowly added to the constant pressure dropping funnel (keep 0 °C reaction conditions), after the dropwise addition, the temperature of the system was slowly raised to room temperature, and the reaction was continued for 12-48 hours with stirring, preferably 18 hours. Stop the reaction, wash with water, extract with DCM three times, collect the organic phase, add anhydrous sodium sulfate to dry, filter sodium sulfate, and rotary evaporate to dry to obtain the product. After further purification with silica gel (ethyl acetate:dichloromethane=8:2), the target product was collected and concentrated by...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com