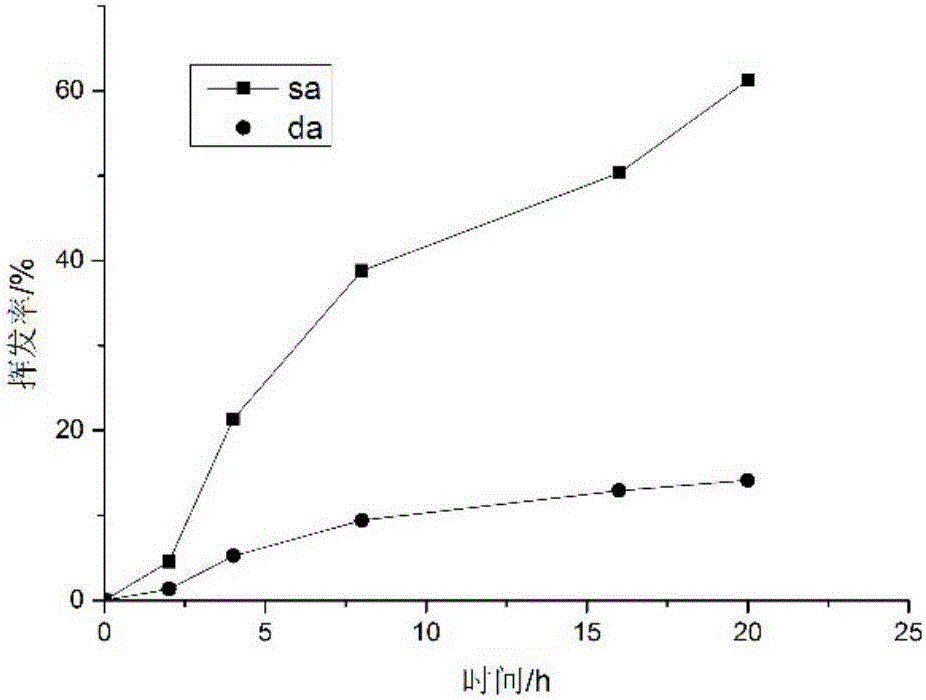
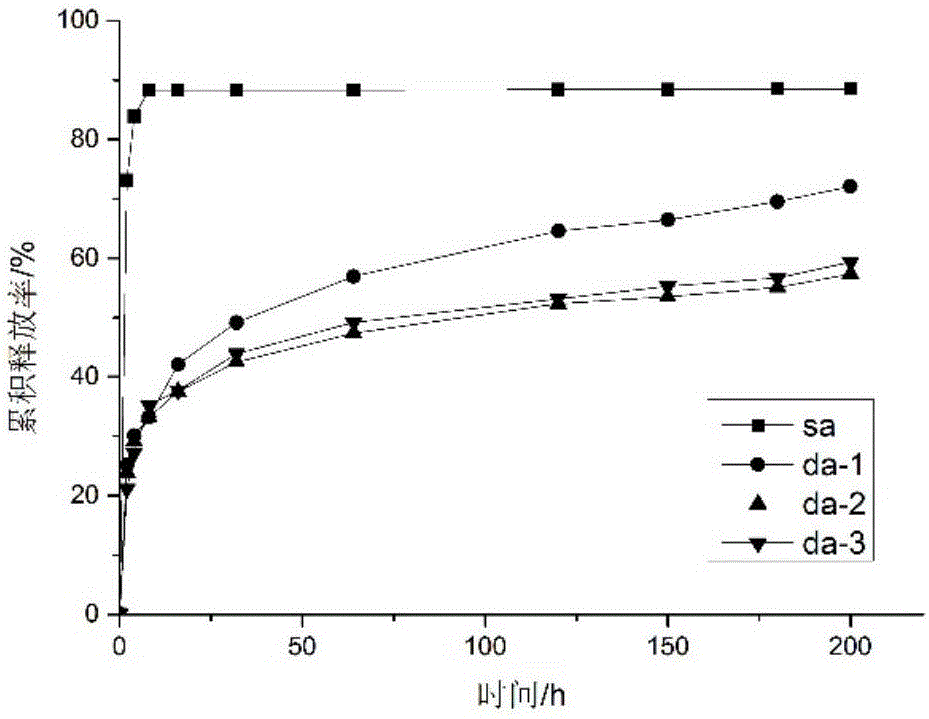
Antibacterial microcapsule with clove oil embedded in laurinol modified alginic acid derivative and preparation method of antibacterial microcapsule
A technology for modifying algae and acid derivatives, applied in microcapsule preparations, microsphere preparation, flexible coverings, etc., can solve the problems of hindering the application of antibacterial packaging materials, volatile clove oil, low water solubility, etc., and achieve enhanced The effect of food shelf life, improving hydrophilicity and improving food quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
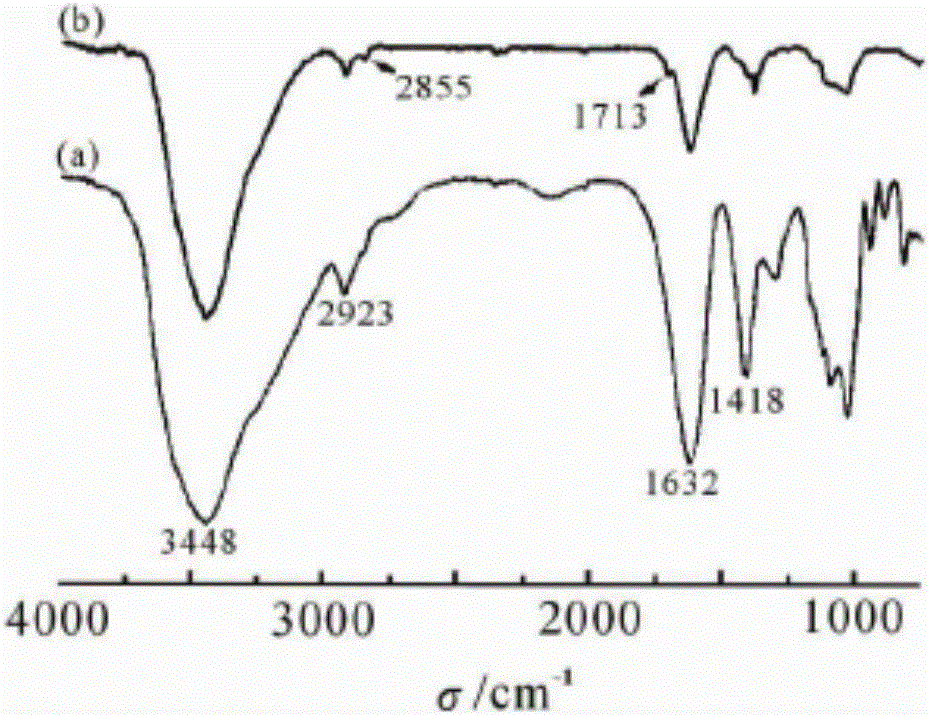
[0026] (1) Preparation of lauryl alcohol modified alginic acid derivative SA-Da:
[0027] a. Prepare a mixed solution according to the volume ratio of formamide:N,N-dimethylformamide 10:9; weigh 1g sodium alginate, put it into 40mL mixed solution, and quickly add 0.5g p-toluenesulfonic acid, stir and acidify 35min; then add 0.4g EDC-HCl and 0.5g 4-dimethylaminopyridine DMAP and stir evenly, and finally add 5g lauryl alcohol, stir and react in a water bath at 55°C for 24h;
[0028] b. Pour the reaction solution obtained in step a into 175 mL of absolute ethanol solution, and rapidly centrifuge for 15 minutes to obtain a precipitate at a centrifugal speed of 5000 rpm; vacuum-dry the precipitate at 0-0.1 MPa, 30-40°C for 20 h, get dry matter;
[0029] c. Dissolve the dried product obtained in step b in 40mL deionized water, stir until completely dissolved, and add NaHCO with a mass concentration of 2%. 3 Adjust the pH of the aqueous solution to neutral; vacuum-dry the obtained ...
Embodiment 2
[0034] (1) Preparation of lauryl alcohol modified alginic acid derivative SA-Da:
[0035] a. Prepare a mixed solution according to the volume ratio of formamide:N,N-dimethylformamide 9:9; weigh 1g sodium alginate, put it into 30mL mixed solution, and quickly add 0.4g p-toluenesulfonic acid, stir and acidify 25min; then add 0.3g EDC-HCl and 0.4g 4-dimethylaminopyridine DMAP and stir evenly, finally add 4g lauryl alcohol, stir and react in a water bath at 50°C for 30h;
[0036] b. Pour the reaction solution obtained in step a into 170 mL of absolute ethanol solution, and rapidly centrifuge for 30 minutes to obtain a precipitate at a centrifugal speed of 4000 rpm; vacuum-dry the precipitate at 0-0.1 MPa, 30-40°C for 30 h, get dry matter;
[0037] c. Dissolve the dried product obtained in step b in 30mL deionized water, stir until completely dissolved, and add NaHCO with a mass concentration of 2%. 3 The pH of the aqueous solution is adjusted to neutral; the obtained neutral sol...
Embodiment 3
[0041] (1) Preparation of lauryl alcohol modified alginic acid derivative SA-Da:
[0042] a. Prepare the mixed solution according to the volume ratio of formamide:N,N-dimethylformamide 10:9; weigh 1g sodium alginate, put it into 40mL mixed solution, and quickly add 0.6g p-toluenesulfonic acid, stir and acidify 45min; then add 0.5g EDC-HCl and 0.6g 4-dimethylaminopyridine DMAP and stir evenly, finally add 6g lauryl alcohol, stir and react in a water bath at 60°C for 20h;
[0043] b. Pour the reaction solution obtained in step a into 170 mL of absolute ethanol solution, and rapidly centrifuge for 20 minutes to obtain a precipitate at a centrifugal speed of 4500 rpm; vacuum-dry the precipitate at 0-0.1 MPa, 30-40°C for 25 hours, get dry matter;
[0044] c. Dissolve the dried product obtained in step b in 50mL deionized water, stir until it is completely dissolved, and add NaHCO with a mass concentration of 2%. 3 Adjust the pH of the aqueous solution to neutral; vacuum-dry the o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| embedding rate | aaaaa | aaaaa |
| embedding rate | aaaaa | aaaaa |
| degree of grafting | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com