Pluronics-PTX (Paclitaxel) amphiphilic macromolecular prodrug and micelle preparation thereof
A paclitaxel and macromolecular technology, applied in the field of medicine and chemical industry, can solve the problems of low solubility of taxane drugs and increase drug safety, and achieve the effects of improving bioavailability, avoiding drug burst release, and good safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
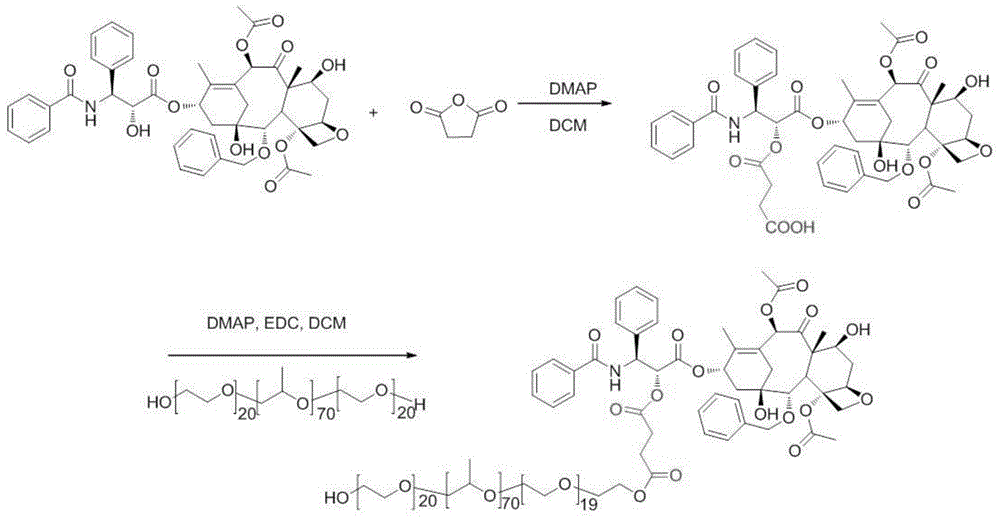
[0044] Embodiment 1 prepares Pluronics-paclitaxel amphiphilic macromolecular prodrug and micellar preparation thereof (synthetic route such as figure 2 shown)
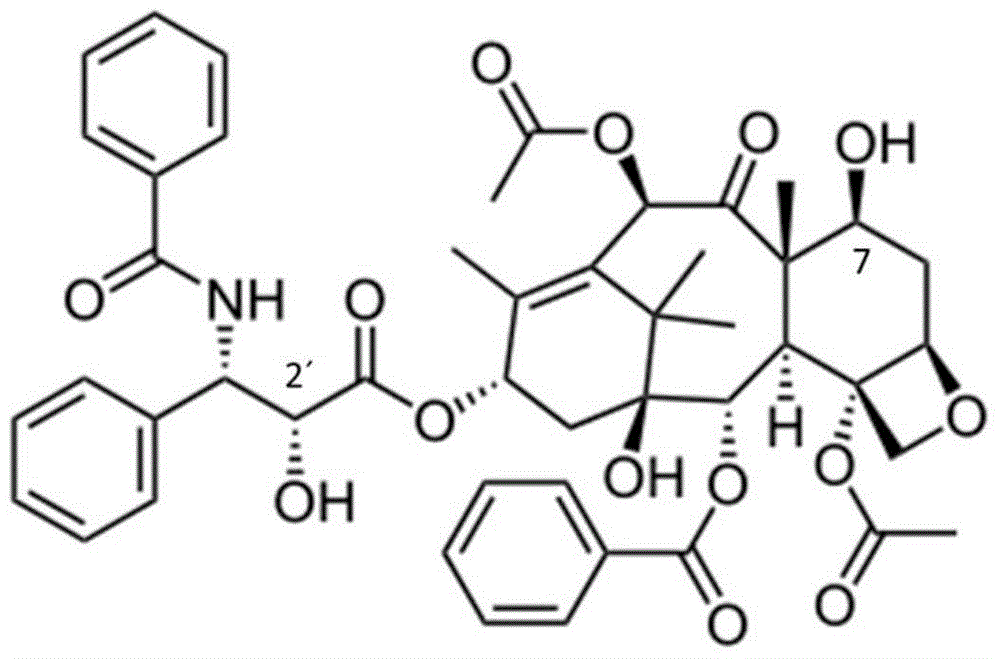
[0045] (1) In a 50mL eggplant-shaped bottle, weigh 300mg paclitaxel and dissolve it in 10mL anhydrous dichloromethane, add succinic anhydride (48mg) and 4-dimethylaminopyridine (DMAP, 30mg) in sequence, under nitrogen protection, room temperature Stir for 24h. Evaporate dichloromethane to dryness, add 10mL ethyl acetate, fully dissolve, then add 10mL dilute hydrochloric acid (mass concentration 10.5%) for pickling, stir for 10min, extract and collect the organic phase, and repeat acid washing twice. The ethyl acetate layer containing the product was dried overnight (12 h) with anhydrous magnesium sulfate, concentrated by rotary evaporation, and the product was separated and purified on a silica gel column by dry loading to obtain carboxy-modified paclitaxel.
[0046] (2) In a 50mL eggplant-shaped bottle, dissolve ca...
Embodiment 2
[0057] (1) In a 50mL eggplant-shaped bottle, dissolve 2g Pluronic P123 and 34mg succinic anhydride in 15mL anhydrous dimethylformamide, then add 0.072mL triethylamine (TEA) and 0.08g DMAP in sequence, and react at room temperature 24h. Put the reaction solution into a treated dialysis bag (molecular weight cut-off 3500Da), put it in 500mL distilled water, dialyze for 48h, change the water every 4h, remove dimethylformamide, and freeze-dry to obtain the carboxyl-terminated Pluronic P123 solid product.
[0058] (2) In a 50mL eggplant-shaped bottle, dissolve 0.5g of Pluronic P123 with terminal carboxyl groups in anhydrous dimethyl sulfoxide, and after the polymer is dissolved, add 0.036g of dicyclohexylcarbodi Imine (DCC), 0.023g DMAP and 0.071g paclitaxel were reacted at room temperature for 48h, and the precipitate generated during the reaction was filtered off. After 72 hours, the water was changed every 4 hours to remove dimethyl sulfoxide, and freeze-dried to obtain the so...
Embodiment 3
[0061] (1) In a 50 mL eggplant-shaped bottle, weigh 100 mg paclitaxel and dissolve in 5 mL anhydrous THF, add maleic anhydride (17 mg) and DMAP (10 mg) in sequence, and stir at room temperature for 36 h under nitrogen protection. Evaporate THF to dryness and remove, add 15mL ethyl acetate, fully dissolve, then add 20mL dilute hydrochloric acid solution for pickling, stir for 30min, extract and collect the organic phase, and repeat acid washing twice. The ethyl acetate layer containing the product was dried overnight with anhydrous magnesium sulfate, concentrated by rotary evaporation, and the product was separated and purified on a silica gel column by dry loading to obtain carboxyl-modified paclitaxel.
[0062] (2) In a 50mL eggplant-shaped bottle, dissolve carboxy-modified paclitaxel (0.2g) in 10mL of anhydrous 1,4-dioxane, and add N,N'-diisopropyl Carbodiimide (DIC) (0.056g), DMAP (0.031g) and Pluronic F68 (2.065g) were reacted at room temperature for 48h. Dialyzed for 72 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com