Sustained-release tablet containing trazodone hydrochloride and preparation method of sustained-release tablet
A technology of trazodone hydrochloride and trazodone acid, which is applied in the field of medicine, can solve the problems of insufficient 24-hour release, unfavorable industrialization promotion of sustained and controlled release drugs, and difficult realization of preparation process requirements, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
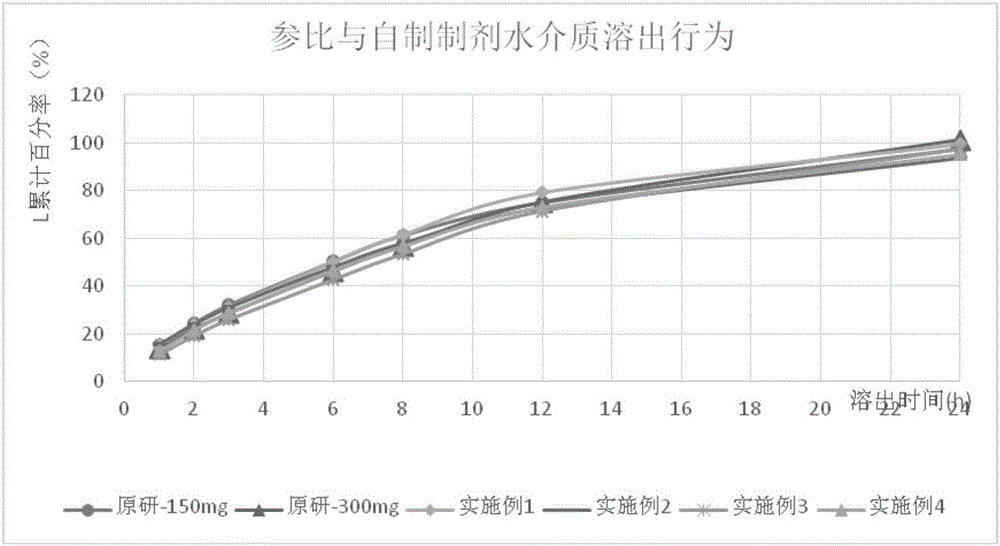
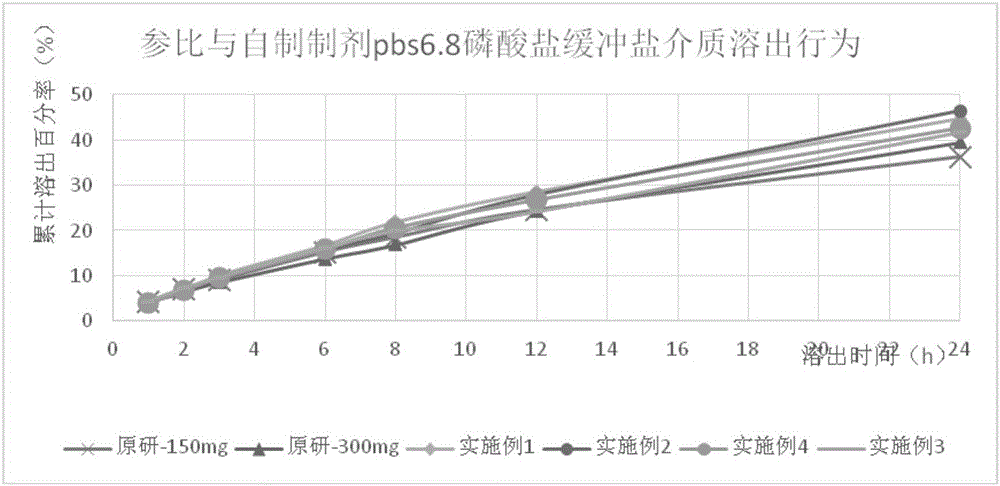
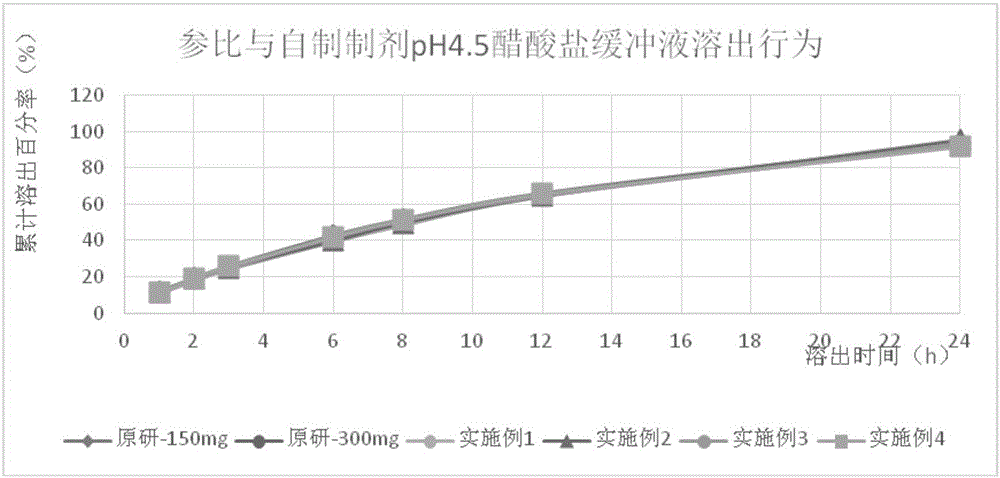
Image
Examples
Embodiment 1
[0047] prescription
[0048]
[0049] Preparation Process:
[0050] Weigh the prescribed amount of trazodone hydrochloride, hypromellose K100M, and direct-pressed lactose for pre-mixing, then add silicon dioxide and magnesium stearate and mix evenly.
[0051] Tablet.
[0052] Coating: Take the prescribed amount of colored film coating agent to prepare a 10% full water coating solution for coating.
Embodiment 2
[0054] prescription
[0055]
[0056]
[0057] Preparation Process:
[0058] Weigh the prescribed amount of trazodone hydrochloride, hypromellose K100M, and direct-pressed lactose for pre-mixing, then add silicon dioxide and magnesium stearate and mix evenly.
[0059] Tablet.
[0060] Coating: Take the prescribed amount of colored film coating agent to prepare a 10% full water coating solution for coating.
Embodiment 3
[0062] prescription
[0063]
[0064]
[0065] Preparation Process:
[0066] Weigh the prescription amount of trazodone hydrochloride, hypromellose (K100M) and lactose for pre-mixing, then add 80% ethanol solution binder to carry out wet 30 mesh granulation, dry at 55±5°C, and then carry out 30 mesh whole grains. Add silicon dioxide and magnesium stearate and mix well.
[0067] Tablet.
[0068] Coating: Take the prescribed amount of colored film coating agent to prepare a 10% full water coating solution for coating.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com