Metformin hydrochloride sustained-release tablet and preparation method thereof
A technology of metformin hydrochloride and sustained-release tablets, applied in the field of medicine, can solve problems such as unfavorable swallowing, high temperature, and sudden release phenomenon, and achieve the effects of overcoming incomplete release, reducing impurity content, and reducing adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
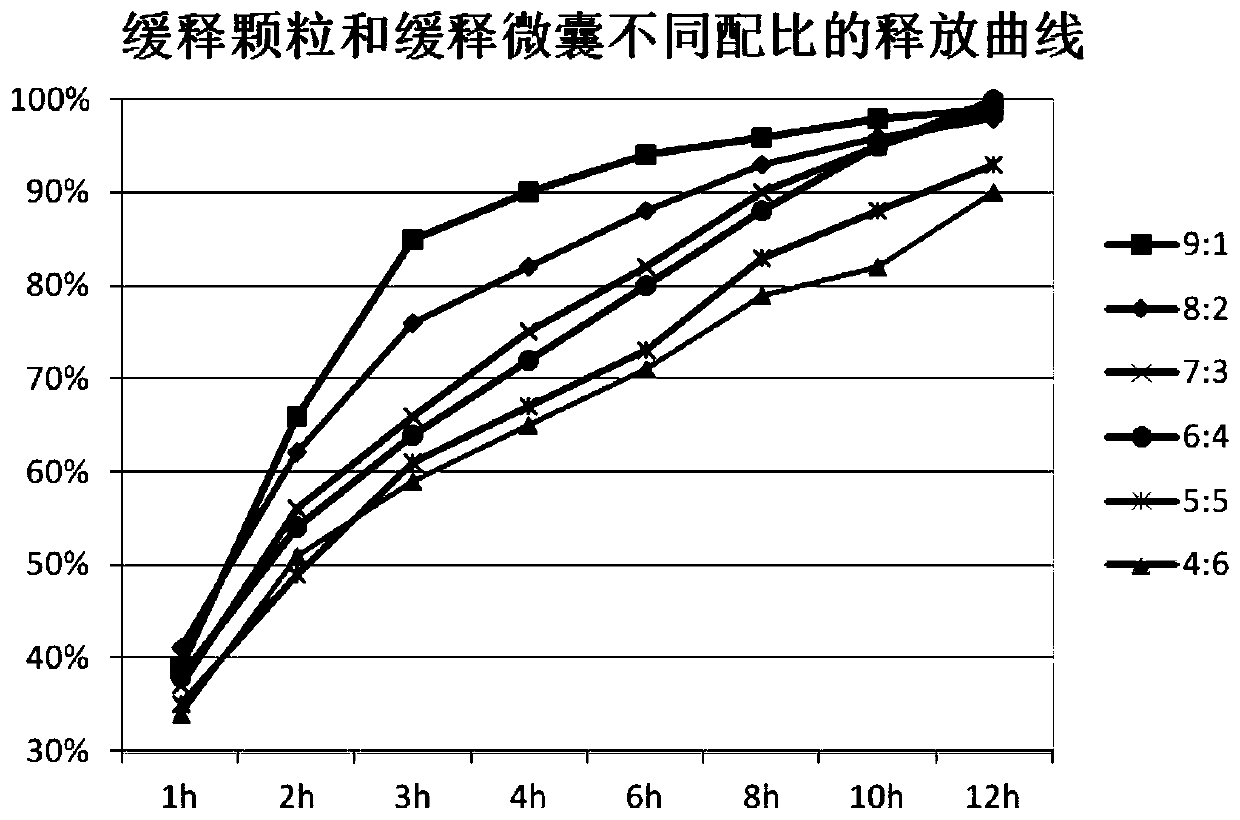
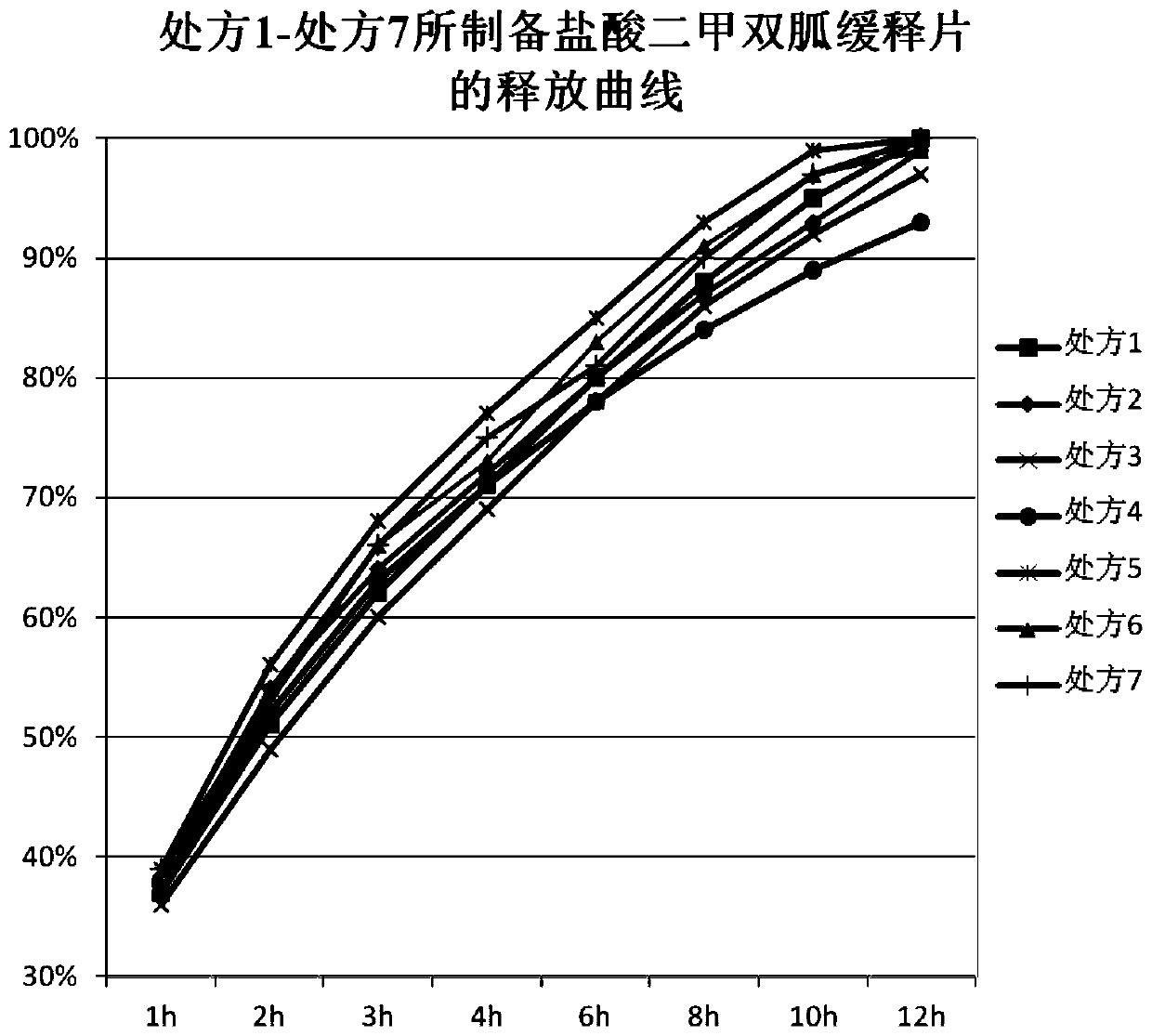
Image
Examples
Embodiment 1
[0063] Embodiment 1 prepares metformin hydrochloride sustained-release tablet of the present invention
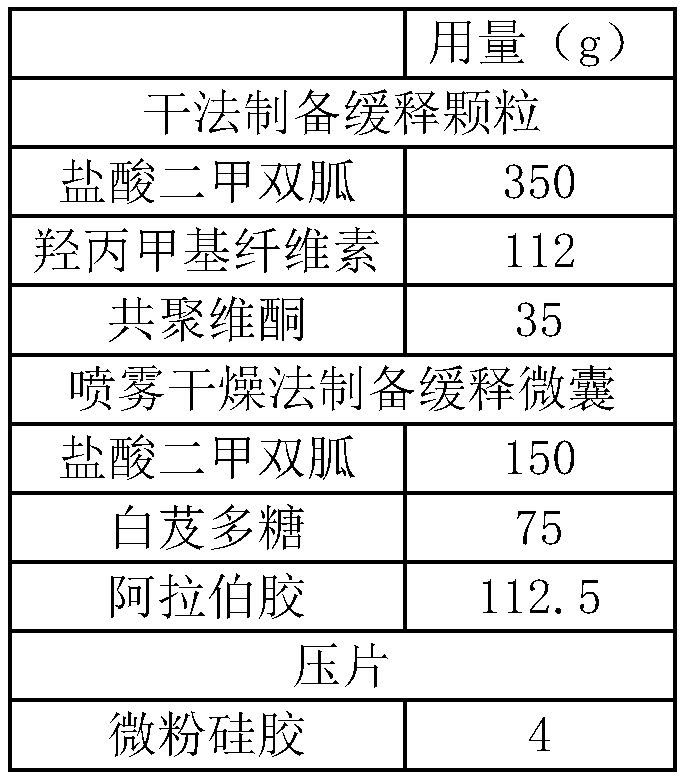
[0064] prescription:
[0065]
[0066] Preparation:
[0067] 1. Preparation of sustained-release granules by dry method:
[0068] a. Mixing: add metformin hydrochloride, hypromellose and copovidone in the prescribed amount into the mixer and mix for 10 minutes;
[0069] b. Dry granulation: transfer the mixed material to a dry granulation roller press for granulation to obtain metformin hydrochloride sustained-release granules.
[0070] 2. Preparation of sustained-release microcapsules by spray drying method:
[0071] a. Prepare bletilla striata polysaccharide solution: weigh the bletilla striata polysaccharide in the prescribed amount, add 470ml of distilled water, heat and stir in a water bath at 50°C to dissolve;
[0072] b. Prepare gum arabic solution: weigh the gum arabic of the prescribed amount, add 938ml of distilled water, heat and stir in a water bath at 50°...
Embodiment 2
[0080] Embodiment 2 prepares metformin hydrochloride sustained-release tablet of the present invention
[0081] prescription:
[0082]
[0083] Preparation:
[0084] 1. Preparation of sustained-release granules by dry method:
[0085] a. Mixing: Add metformin hydrochloride, hypromellose and microcrystalline cellulose in the prescribed amount into the mixer and mix for 10 minutes;
[0086] b. Dry granulation: transfer the mixed material to a dry granulation roller press for granulation to obtain metformin hydrochloride sustained-release granules.
[0087] 2. Preparation of sustained-release microcapsules by spray drying method:
[0088] a. Prepare bletilla striata polysaccharide solution: weigh the bletilla striata polysaccharide in the prescribed amount, add 330ml of distilled water, heat and stir in a water bath at 50°C to dissolve;
[0089] b. Preparation of gum arabic solution: weigh the gum arabic of the prescribed amount, add 910ml of distilled water, heat and stir...
Embodiment 3
[0097] Embodiment 3 prepares metformin hydrochloride sustained-release tablet of the present invention
[0098] prescription:
[0099]
[0100] Preparation:
[0101] 1. Preparation of sustained-release granules by dry method:
[0102] a. Mixing: Add metformin hydrochloride, hypromellose and microcrystalline cellulose in the prescribed amount into the mixer and mix for 10 minutes;
[0103] b. Dry granulation: transfer the mixed material to a dry granulation roller press for granulation to obtain metformin hydrochloride sustained-release granules.
[0104] 2. Preparation of sustained-release microcapsules by spray drying method:
[0105] a. Prepare bletilla striata polysaccharide solution: weigh the bletilla striata polysaccharide in the prescribed amount, add 265ml of distilled water, heat and stir in a water bath at 50°C to dissolve;
[0106] b. Preparation of gum arabic solution: weigh the gum arabic of the prescribed amount, add 1100ml of distilled water, heat and sti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com