Nifedipine sustained-release tablet and production process thereof
A technology for nifedipine and nifedipine, which is applied in the field of medicine, can solve the problems of uneven drug dose dispersion, unstable sustained release, easy burst release and the like, so as to avoid burst release phenomenon, uniform drug dose dispersion and lasting curative effect. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
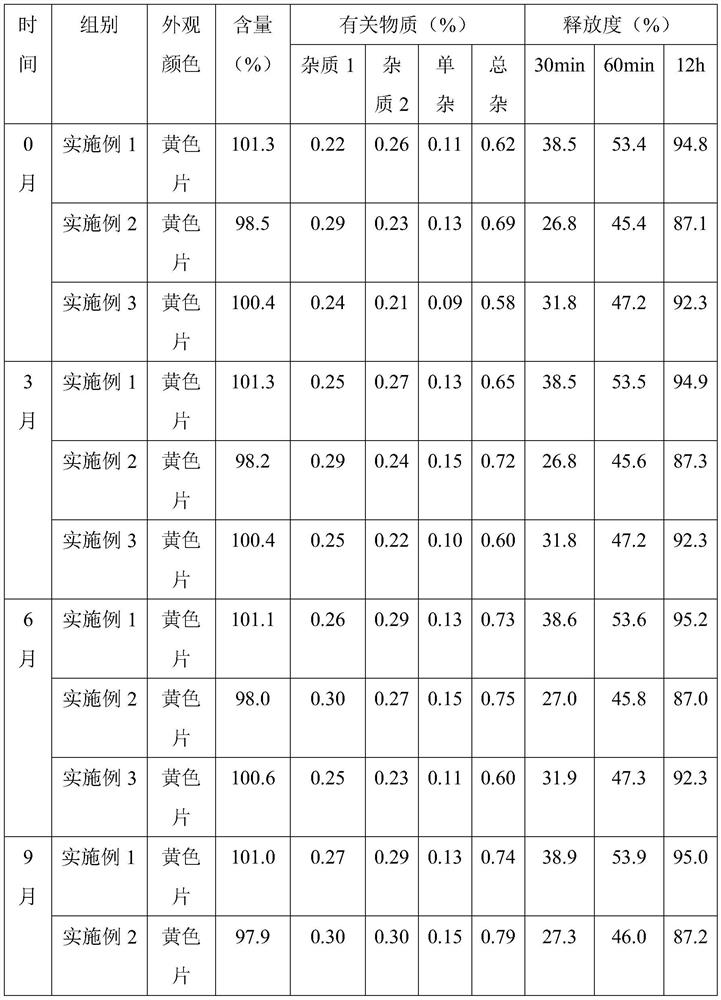
Embodiment 1
[0034] Embodiment 1, a kind of nifedipine sustained-release tablet
[0035] The nifedipine sustained-release tablet comprises the following components and parts by mass thereof:
[0036] 20 parts of nifedipine, 13 parts of slow-release agent, 3 parts of blocking agent, 72 parts of filler, 1 part of disintegrating agent, 0.3 part of glidant, 0.5 part of lubricant; the slow-release agent is hypromellose base cellulose; the blocker is composed of sodium carboxymethyl cellulose and chitosan oligosaccharide with an average molecular weight of 3000 in a mass ratio of 7:3; the filler is microcrystalline cellulose; the disintegrant is cross-linked Lipovidone; the glidant is composed of magnesium stearate and silicon dioxide in a mass ratio of 1:4; the lubricant is glyceryl monostearate.
[0037] The production technology of described nifedipine slow-release tablet is:
[0038] S1 Grinding nifedipine to a particle size D90 of 10 μm to obtain nifedipine powder;
[0039] S2 Pulverize ...
Embodiment 2
[0043] Embodiment 2, a kind of nifedipine slow-release tablet
[0044] The nifedipine sustained-release tablet comprises the following components and parts by mass thereof:
[0045] 20 parts of nifedipine, 20 parts of slow-release agent, 5 parts of blocker, 76 parts of filler, 2 parts of disintegrant, 0.7 part of glidant, 1 part of lubricant; the slow-release agent is hypromellose base cellulose; the blocker is composed of sodium carboxymethylcellulose and chitosan oligosaccharide with an average molecular weight of 3000 in a mass ratio of 10:2; the filler is microcrystalline cellulose; the disintegrant is carboxymethyl cellulose Calcium methylcellulose; the glidant is composed of magnesium stearate and silicon dioxide in a mass ratio of 1:6; the lubricant is talcum powder.
[0046] The production technology of described nifedipine slow-release tablet is:
[0047] S1 Grinding nifedipine to a particle size D90 of 50 μm to obtain nifedipine powder;
[0048] S2 Pulverize the s...
Embodiment 3
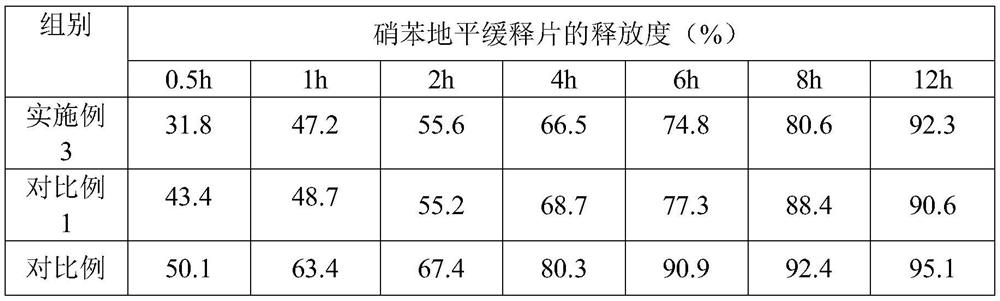
[0052] Embodiment 3, a kind of nifedipine sustained-release tablet
[0053] The nifedipine sustained-release tablet comprises the following components and parts by mass thereof:
[0054] 20 parts of nifedipine, 18 parts of slow-release agent, 4 parts of blocker, 74 parts of filler, 1.2 parts of disintegrant, 0.5 part of glidant, 0.6 part of lubricant; base cellulose; the blocker is composed of sodium carboxymethyl cellulose and chitosan oligosaccharide with an average molecular weight of 3000 in a mass ratio of 8:3; the filler is microcrystalline cellulose; the disintegrant is composed of Lipovidone and sodium lauryl starch are composed of 5:2 by mass ratio; the glidant is composed of magnesium stearate and silicon dioxide by mass ratio of 1:5; the lubricant has an average molecular weight of 2000 of polyethylene glycol.
[0055] The production technology of described nifedipine slow-release tablet, step is as follows:
[0056] S1 Grinding nifedipine to a particle size D90 of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com