Biologically responsive nitric oxide donor type polymer prodrug and preparation method thereof
A body-shaped polymer and nitric oxide technology, applied in drug combinations, pharmaceutical formulations, medical preparations of non-active ingredients, etc., can solve the problems of limiting the application of small molecule chemotherapy drugs, large toxic and side effects in healthy tissues, and poor drug targeting, etc. problem, to achieve the effect of improving the tumor microenvironment, less toxic and side effects, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
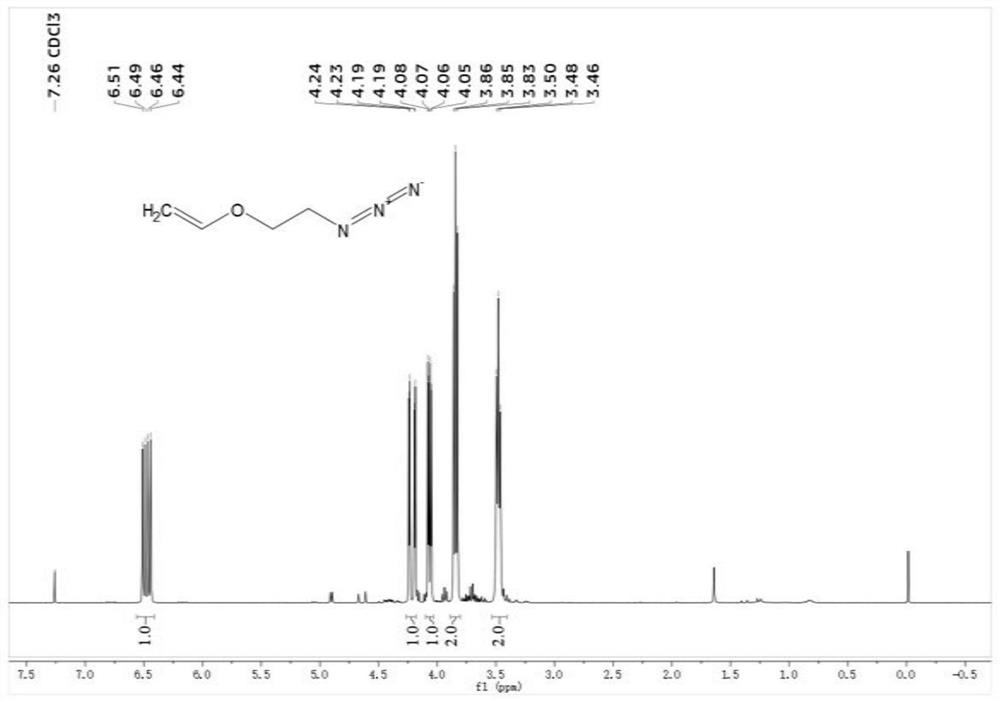
[0044] (1) Synthesis of small molecule 2-azaethyl vinyl ether (AzVE)
[0045] The synthetic route of small molecule AzVE is as follows:
[0046]
[0047] Weigh 2-chloroethyl vinyl ether (29.5mL, 0.290mol) and dissolve it in 175mL of anhydrous N, N-dimethylformamide, in N 2 Under protective conditions, sodium azide (21.2 g, 0.326 mol) was slowly added, reacted at 80°C for 5 h, and then distilled under reduced pressure to obtain 2-azaethyl vinyl ether (AzVE) as a colorless transparent liquid, producing Rate 72%, H NMR spectrum such as figure 1 shown.
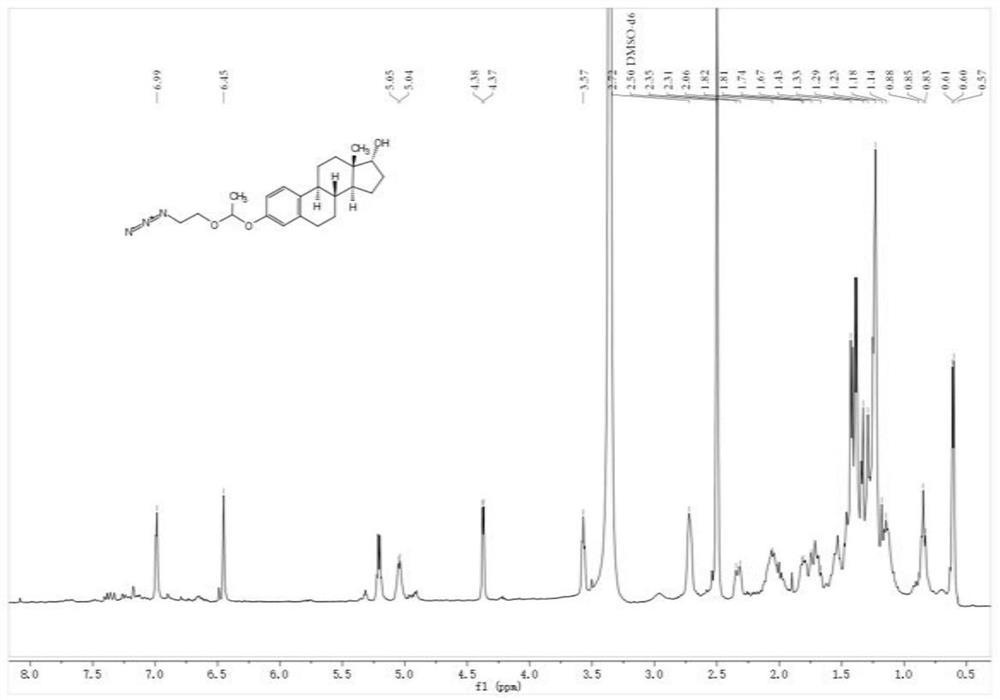
[0048] (2) Synthesis of small molecule prodrug estradiol-2-azaethyl vinyl ether complex (Estradiol-AzVE)
[0049] The synthetic route of the small molecule Estradiol-AzVE is as follows:
[0050]
[0051] Take 2-azaethyl vinyl ether (0.5g, 4.4mmol) and estradiol (120mg, 0.44mmol) respectively in a reaction flask, add an appropriate amount of dichloromethane solvent, and add p-toluenesulfonic acid under nitrogen protection...
Embodiment 2
[0056] Example 2 Preparation of bioresponsive nitric oxide donor polymer estradiol prodrug micelles (PEG-PNTC-co-Estradiol)
[0057] Polymer prodrug micelles (PEG-PNTC-co-Estradiol) were prepared by solvent exchange method. Slowly add 0.1 mL of N,N-dimethylformamide solution (20 mg / mL) of polymer prodrug PEG-PNTC-co-Estradiol to 1 mL of high-purity water under ultrasonic conditions, and continue to sonicate the resulting mixed solution for half an hour , and finally dialyzed in high-purity water for 2 h. Figure 4 is the particle size characterization diagram of polymer prodrug micelles (PEG-PNTC-co-Estradiol). The results showed that the average particle size of the prodrug micelles was 103nm, and the PDI was 0.21.
Embodiment 3
[0058] Example 3 In vitro degradation experiment of bioresponsive polymer estradiol prodrug micelles (PEG-PNTC-co-Estradiol)
[0059] Prepare two parts of polymer prodrug micelles (PEG-PNTC-co-Estradiol, 1mL, 1mg / mL) and add them to the glass sample cell, add a certain amount of hydrochloric acid solution to one of the sample cells to make the final pH of the micellar solution 5.0, add an equal amount of pH 7.4 phosphate buffer solution to another sample cell to make the final pH of the micellar solution 7.4, then seal the glass sample cell with a rubber stopper, shake it evenly, and place it in a constant temperature shaker (200rpm) at 37°C. At selected times at 37°C, the particle size change of the particles was tracked by dynamic laser light scattering (DLS). Figure 5 It is the particle size diagram of the polymer prodrug micelle (PEG-PNTC-co-Estradiol) placed under different pH conditions for different times. The results showed that at pH 7.4, the particle size of the pr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com