Trimetazidine hydrochloride sustained release tablet and preparation method thereof
A technology of trimetazidine hydrochloride and sustained-release tablets, which is applied in the direction of pharmaceutical formulas, medical preparations containing no active ingredients, and medical preparations containing active ingredients, etc., which can solve the problems of pain relief, curative effect and clinical application limitations, heart disease, etc. Function damage and other problems, to achieve the effect of improving compliance, avoiding sudden release phenomenon, and stable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
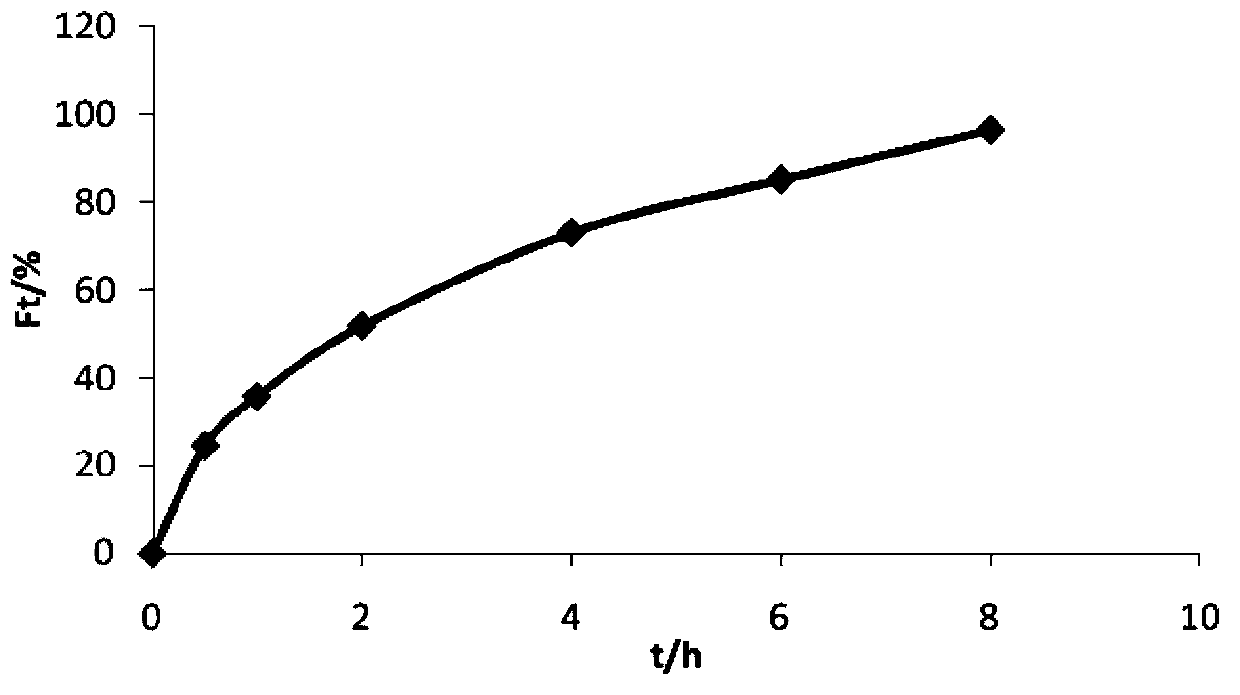
Embodiment 1
[0032] Prepare 1000 trimetazidine hydrochloride sustained-release tablets according to the prescription in the following table 1 and the above-mentioned preparation method.
[0033] Table 1 Prescription composition and dosage of trimetazidine hydrochloride sustained-release tablets (based on 1000 tablets)
[0034]
[0035] In the table, 8% povidone-80% ethanol-water solution obtains by following preparation method: be that in the ethanol aqueous solution of 80% that povidone is dissolved in mass concentration, the mass concentration that adjusts povidone is 8%, namely have to. Adhesives in the following examples were prepared using the same method.
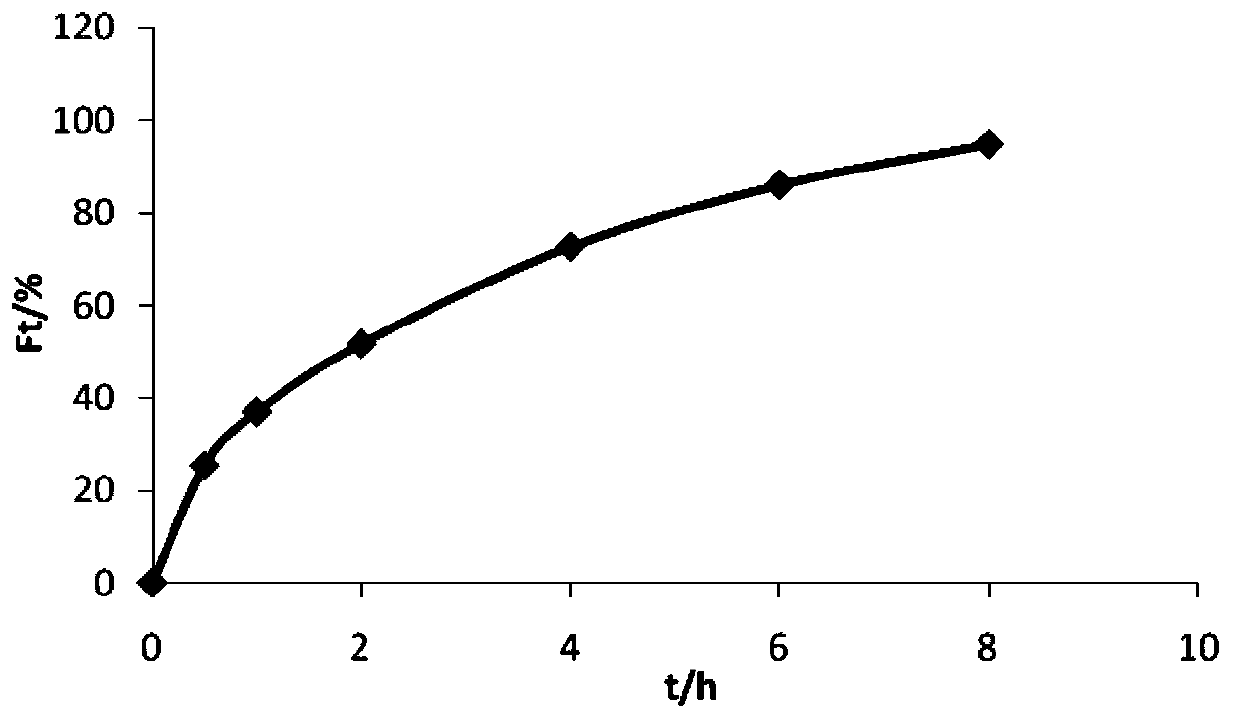
Embodiment 2
[0037] Prepare 1000 trimetazidine hydrochloride sustained-release tablets according to the prescription in the following table 2 and the above-mentioned preparation method.
[0038] Table 2 Prescription composition and dosage of trimetazidine hydrochloride sustained-release tablets (based on 1000 tablets)
[0039]
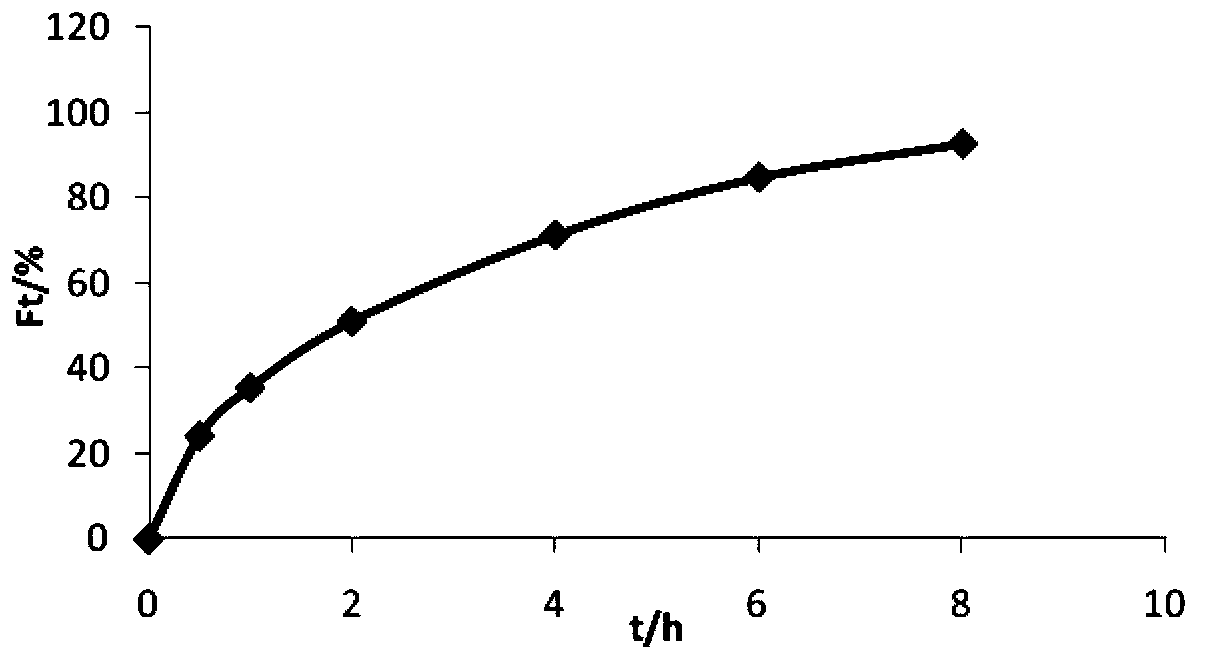
Embodiment 3
[0041] Prepare 1000 trimetazidine hydrochloride sustained-release tablets according to the prescription in the following table 3 and the above-mentioned preparation method.
[0042] Table 3 Prescription composition and dosage of trimetazidine hydrochloride sustained-release tablets (based on 1000 tablets)
[0043]
[0044]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com