Preparation method of controlled-release drug
A technology for controlled release of drugs and active drugs, which is applied in the fields of pharmaceutical formulations, drug delivery, and active ingredients of heterocyclic compounds. It can solve the problems of difficult to achieve large-scale production promotion and application, and it is difficult to achieve osmotic pumps with ordinary equipment, so as to achieve easy scale Production and marketing, avoiding sudden release, safe and effective clinical application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
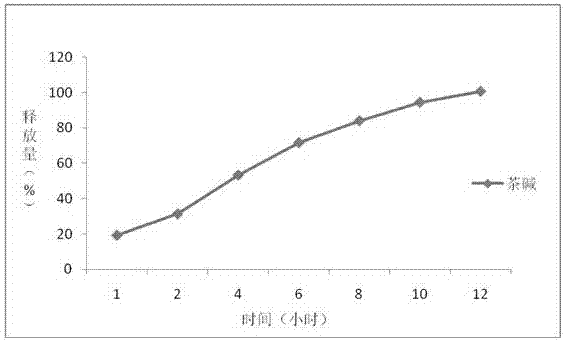
Embodiment 1
[0038] The preparation of embodiment 1 theophylline controlled release tablet
[0039] (1) According to the components and consumption in Table 1, at first take theophylline bulk drug and ethyl cellulose, polyethylene glycol, hydroxypropyl methyl cellulose, hydrogenated oil, talcum powder, magnesium stearate;
[0040] (2) Add theophylline bulk drug, ethyl cellulose, hydroxypropyl methyl cellulose, hydrogenated oil, talcum powder, and magnesium stearate into a three-dimensional mixer and mix for 10 minutes, and take out the material;
[0041] (3) Take out the mixed materials and prepare for the hot-melt extrusion process. Set the temperature of the hot-melt extrusion equipment to 155~175°C and start hot-melting. Initially set the screw speed to 10rpm~30rpm. Accelerate according to the actual situation;
[0042] (4) Long strips are obtained after hot melt extrusion. During the extrusion process, the cutter on the hot melt extruder can be used, or a crushing and granulator can b...
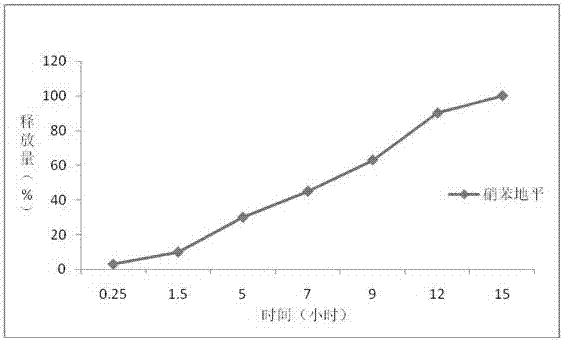
Embodiment 2
[0049] Example 2 Preparation of Nifedipine Controlled-release Tablets
[0050] (1) According to the components and amounts in Table 2, first weigh nifedipine, ethylene-vinyl acetate copolymer, povidone, sodium carmellose, colloidal silicon dioxide and silicon dioxide.
[0051] (2) Nifedipine is mixed with colloidal silicon dioxide, and then mixed with ethylene-vinyl acetate copolymer, povidone, sodium carboxymethylcellulose, and silicon dioxide;
[0052] (3) Set the temperature range of the hot melt extruder to 170~180°C, set the initial screw speed to 10rpm, and then adjust it according to the extrusion state;
[0053] (4) The hot-melt extruded strip-shaped object is covered with a layer of ethyl cellulose powder on the surface of the extruded object after it has just exited the hot-melt extruder, and the hot-melt basic equipment is used to Cutter to crush the long sticks into short sticks with a length of 1 mm to 1.5 mm;
[0054] (5) Add lactose monohydrate and microcrysta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| angle of repose | aaaaa | aaaaa |
| relative humidity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com