Memantine hydrochloride slow-release pellet preparation and preparation method thereof
A technology of memantine hydrochloride and sustained-release pellets, which is applied in the direction of bulk delivery, active ingredients of amines, nervous system diseases, etc., can solve the problems of sudden release risks and safety hazards of sustained-release tablets, and achieve long-lasting and stable drug effects. Increased safety and good fluidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
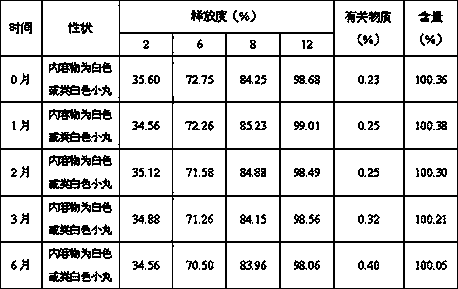
[0020] Embodiment 1: Preparation of memantine hydrochloride sustained-release pellets
[0021] Prescription: weight of raw and auxiliary materials (g)
[0022] Blank core 799
[0023] Memantine Hydrochloride 56
[0024] Hypromellose 110
[0026] Polyethylene glycol 10
[0027] 95% ethanol in appropriate amount.
[0028] Preparation method: 1) Dissolve hypromellose in 95% ethanol, and at the same time dissolve memantine hydrochloride and polyethylene glycol in 95% ethanol and add it;
[0029] 2) Put the blank pellet core in the coating machine, spray the drug sustained-release composite layer solution prepared in step 1) on the blank pellet core after preheating, and control the air inlet temperature at 40°C to prepare drug-containing pellets;
[0030] 3) Dry the drug-containing pellets obtained in step 2) in a hot air circulation oven at 60°C to 70°C for 2 hours to obtain sustained-release pellets; , to obtain sustained-release pellets.
[0031] ...
Embodiment 2
[0033] prescription
[0034] Raw materials Weight (g)
[0035] Blank core 789
[0036] Memantine Hydrochloride 56
[0037] Stearic acid 120
[0038] Povidone 10
[0040] 95% ethanol in appropriate amount.
[0041] Preparation Process:
[0042] (1) Fully dissolve the stearic acid, memantine hydrochloride and povidone in the prescription with 95% ethanol, and stir evenly;
[0043] (2) Put the blank pellet core in the coating machine, spray the drug sustained-release composite layer solution prepared in step (1) on the blank pellet core after preheating, and control the air inlet temperature at 40°C to prepare drug-containing pellets;
[0044] (3) Dry the drug-containing pellets obtained in step (2) in a hot air circulation oven at 60°C to 70°C for 2 hours to obtain sustained-release pellets;
[0045] The sustained-release pellets are packed into hollow capsules to obtain memantine hydrochloride sustained-release pellets capsules.
Embodiment 3
[0047] prescription
[0048] Raw materials Weight (g)
[0049] Blank core 799
[0050] Memantine Hydrochloride 56
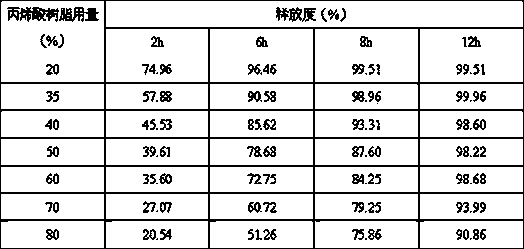
[0051] Acrylic resin (Utech NE30D) 110
[0052] Triethyl citrate 5
[0053] 95% ethanol appropriate amount
[0054] Preparation Process:
[0055] (1) Fully dissolve the stearic acid, memantine hydrochloride and povidone in the prescription with 95% ethanol, and stir well.
[0056] (2) Put the blank pellet core in the storage tank of the fluidized boiling coating machine, pass hot air from the bottom to make it in a fluidized state, and spray the drug sustained-release compound solution in step (1) through the bottom Spray in so the solution is sprayed onto blank pellet cores and allowed to dry.
[0057](3) The working parameters of the fluidized boiling coating machine are: the air supply temperature is set to 35~60°C; the fan frequency is set to 40~50hZ; the nozzle atomization pressure is 1.5~3.0bar.
[0058] The sustained-release pellets are packed into...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com