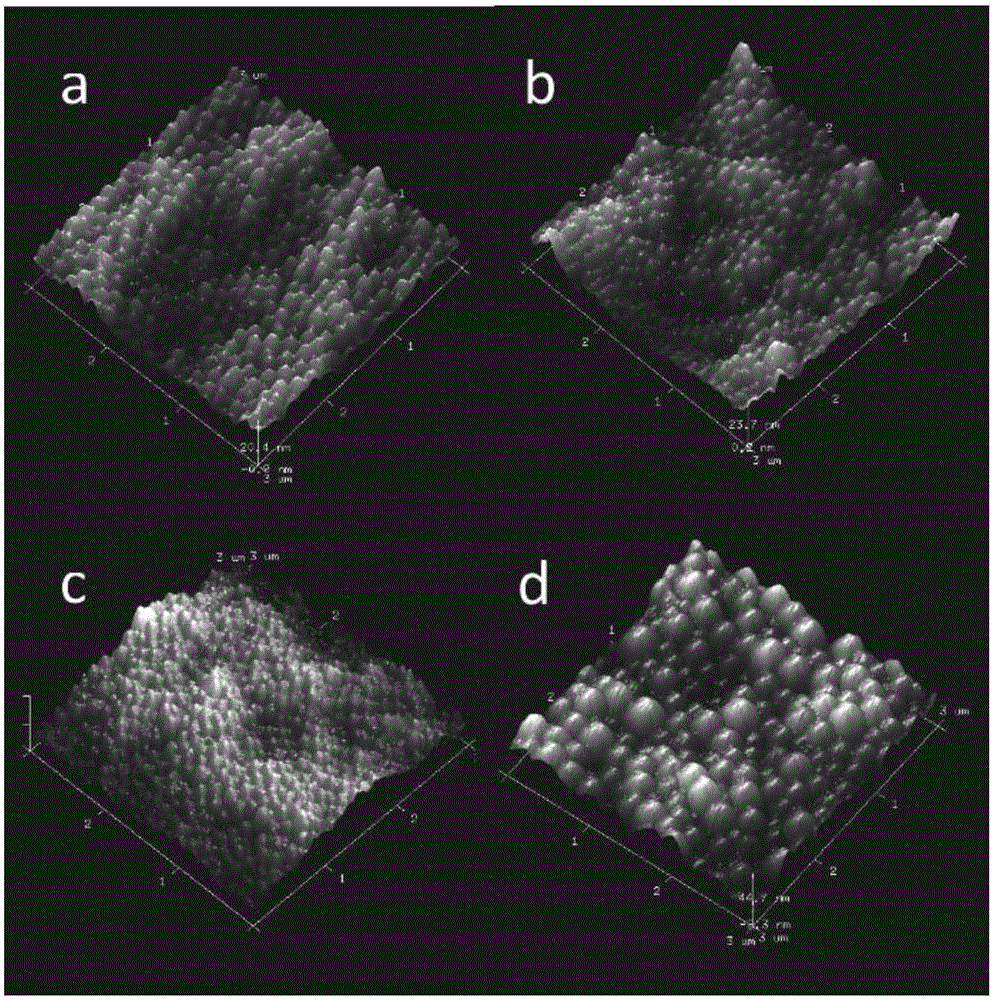
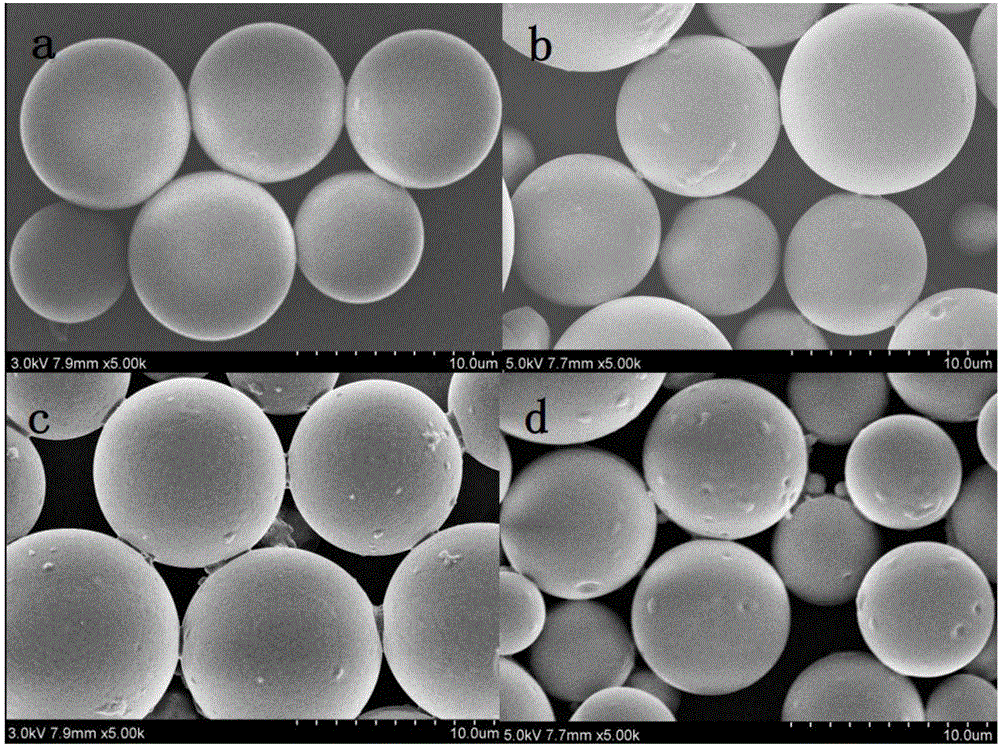
PLGA (Poly Lactic-co-Glycolic Acid)-gelatin composite microspheres carrying genistein and preparation method thereof
A technology of genistein and composite microspheres, which can be applied to medical preparations without active ingredients, medical preparations containing active ingredients, and drug combinations, etc., can solve the problem of uneven liquid force, uneven particle size, and equipment cost. Low-level problems, to avoid spherical irregularity and size increase, the effect of orderly binding of peptide chains
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] 1) Take 500mg of type A gelatin (with an isoelectric point of 7-9) and add it to 10mL of deionized water, heat and dissolve to make an aqueous gelatin solution; then add 10mL of acetone to the aqueous gelatin solution to obtain precipitated gelatin;
[0036] 2) Take 240 mg of the precipitated gelatin obtained in step 1), and then redissolve it with 10 mL of deionized water to obtain an aqueous gelatin solution, and use hydrochloric acid and sodium hydroxide to adjust the pH value of the solution to 2.5;
[0037] 3) Add 30 mL of acetone dropwise to the gelatin aqueous solution obtained in step 2) under 500 rpm magnetic stirring to emulsify the system; then immediately add 40 μL of 50% glutaraldehyde aqueous solution to the above system, and continue Stir for 1 hour, then let the emulsion stand at room temperature for 8 hours to solidify the gelatin nanoparticles, and refrigerate at 4°C;
[0038] 4) Take 4mL of the solution prepared in step 3) and centrifuge at 6000rpm, r...
Embodiment 2
[0043] 1) Add 500 mg of type A gelatin to 15 mL of deionized water, heat and dissolve to make an aqueous gelatin solution; then add 15 mL of acetone to the aqueous gelatin solution to obtain precipitated type A gelatin;
[0044] 2) Take 200 mg of the precipitated gelatin obtained in step 1), and then dissolve it again with 15 mL of deionized water to obtain an aqueous gelatin solution, and adjust the pH value of the solution to 3.0;
[0045] 3) Add 40 mL of acetone dropwise to the gelatin aqueous solution obtained in step 2) under magnetic stirring at 750 rpm to emulsify the system; then immediately add 40 μL of 50% glutaraldehyde aqueous solution to the above system, and continue Stir for 1.5 hours, then let the emulsion stand at room temperature for 10 hours to solidify the gelatin nanoparticles, and refrigerate at 4°C;
[0046] 4) Take 2 mL of the solution prepared in step 3) and centrifuge at 6000 rpm, remove the supernatant, wash the precipitate with deionized water 4 tim...
Embodiment 3
[0051] 1) Add 500mg of type A gelatin to 15mL of deionized water, heat and dissolve to make an aqueous gelatin solution; then add 20mL of acetone to the aqueous gelatin solution to obtain precipitated type A gelatin;
[0052] 2) Take 270 mg of the precipitated gelatin obtained in step 1), and then dissolve it again with 20 mL of deionized water to obtain an aqueous gelatin solution, and adjust the pH value of the solution to 3.5;
[0053] 3) Add 40 mL of acetone dropwise to the gelatin aqueous solution obtained in step 2) under 1000 rpm magnetic stirring to emulsify the system; then immediately add 50 μL of glutaraldehyde aqueous solution with a mass fraction of 50% to the above system, and continue Stir for 1 hour, then let the emulsion stand at room temperature for 12 hours to solidify the gelatin nanoparticles, and refrigerate at 4°C;
[0054] 4) Take 2 mL of the solution prepared in step 3) and centrifuge at 7000 rpm, remove the supernatant, wash the precipitate with deioniz...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com