Slow release drop pills comprising toraesmide active ingredient and method for preparing same
A technology of torasemide and sustained-release pellets, which is applied in the direction of active ingredients of heterocyclic compounds, bulk delivery, blood diseases, etc., can solve problems such as major side effects, achieve easy industrialization, improve therapeutic effect, and increase safety Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
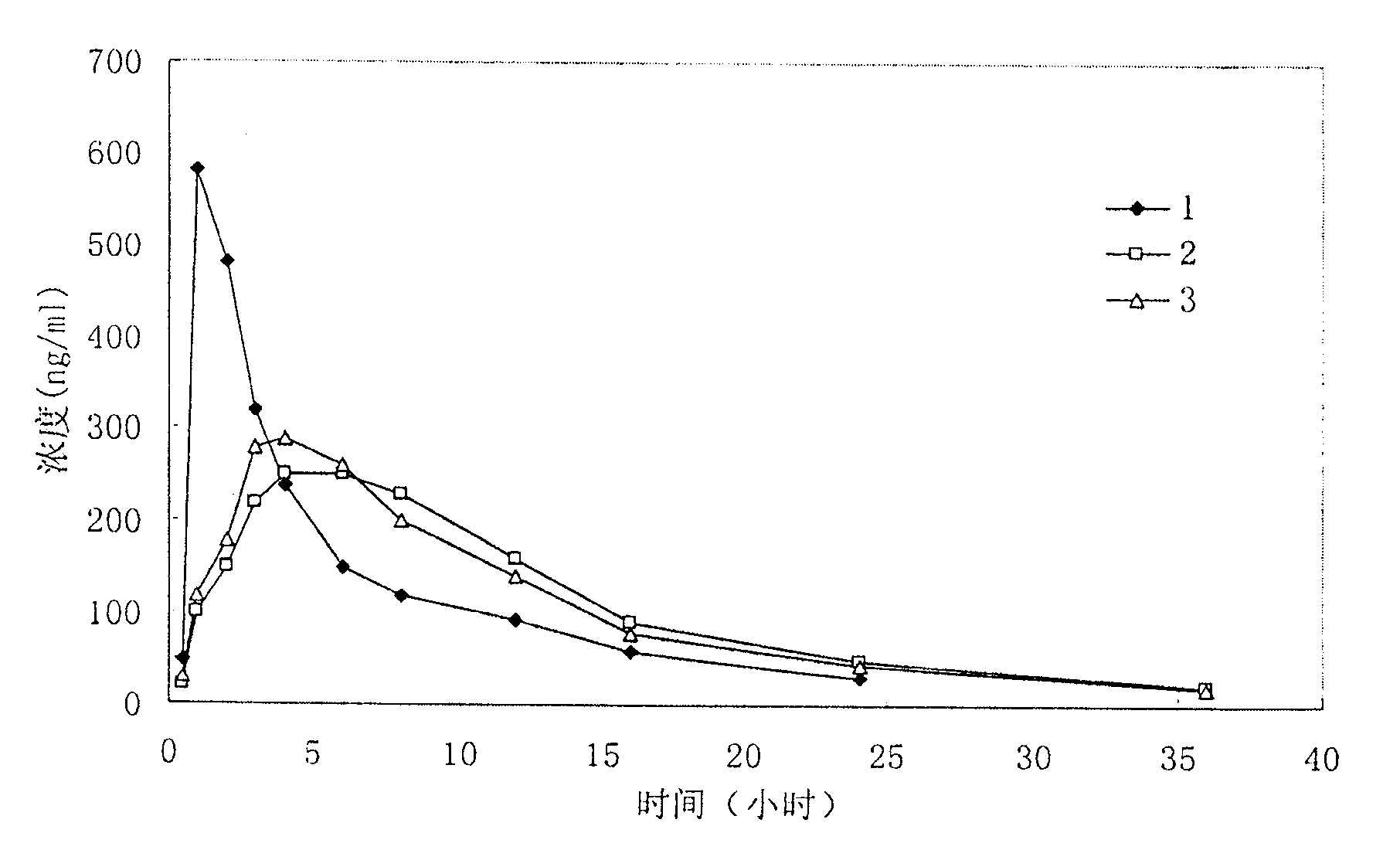
Examples
Embodiment 1
[0045] Embodiment 1, the preparation of drug-containing pellets
[0046]
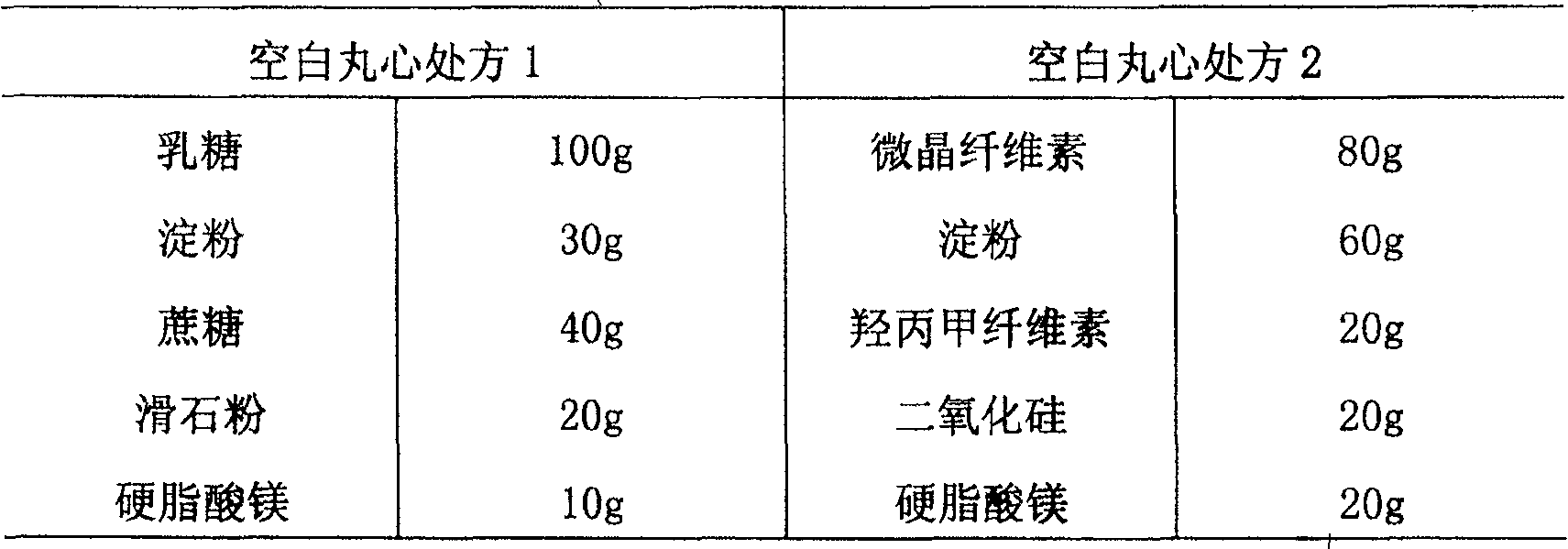
[0047] Mix the prescription amount of lactose (or microcrystalline cellulose) and starch evenly, add the binder prepared by sucrose or hypromellose, use a granulator (or coating pan) to start and roll the pellets to the required The mesh number is 15-30 mesh (lubricant and glidant are added while rolling the pellets), dried, and the best 18-25 mesh is screened out.
[0048]
[0049] Preparation:
[0050] Add torsemide, hypromellose (or polyvinylpyrrolidone) and talcum powder described in the prescription into water or 70% ethanol respectively, and stir for 20 minutes to form a uniform mixture. In the fluidized bed (or coating pan), keep the temperature of the pellets at 40°C-60°C, spray the mixed solution evenly on the outer layer of the blank pellets obtained in the previous step to prepare drug-containing pellets.
Embodiment 2
[0051] Embodiment 2, preparation of drug-containing pellets
[0052]
[0053]
[0054] Preparation
[0055] Mix the prescription amount of lactose (or microcrystalline cellulose), starch and medicine evenly, add sucrose and polyethylene glycol or (hypromellose and polyethylene glycol) prepared binder, and use a granulator ( or coating pan) and roll the balls to the required mesh number of 15-30 (adding lubricant and glidant while rolling the balls), sieve out the best 18-25 mesh, and dry to get.
Embodiment 3
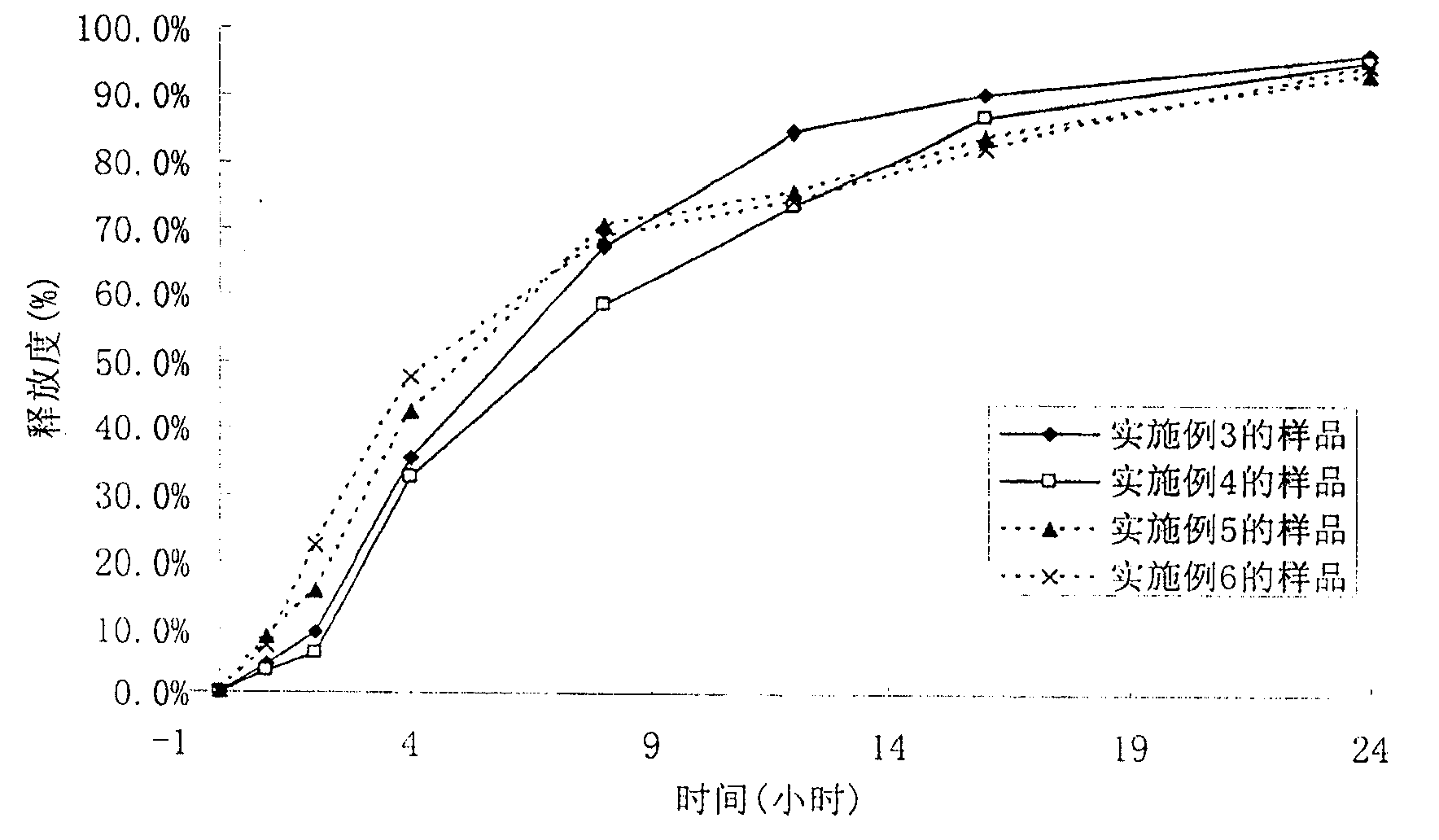
[0056] Embodiment 3, the preparation of sustained-release pellets
[0057] prescription
[0058] The drug-containing pellet 150g of embodiment 1
[0059] Acrylic resin NE30D 20g
[0060] Talc powder 5g
[0061] water 150ml
[0062] Preparation:
[0063] The pellets obtained in Example 1 were put into a fluidized bed (or coating pan), and the temperature of the pellets was kept at 25°C. Add triethyl citrate and talcum powder to the acrylic resin NE30D aqueous dispersion, stir evenly, and spray on the outer layer of drug-containing pellets at a flow rate of 5ml / min to make sustained-release pellets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com