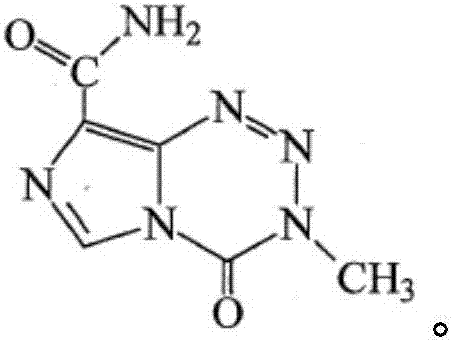
Temozolomide pharmaceutical composition as well as preparation method and application thereof
A technology for temozolomide and composition, applied in the field of pharmaceutical compositions, can solve the problems of affecting clinical efficacy, poor preparation content uniformity, poor fluidity, etc., and achieve the effects of improving drug efficacy, improving stability, and improving dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
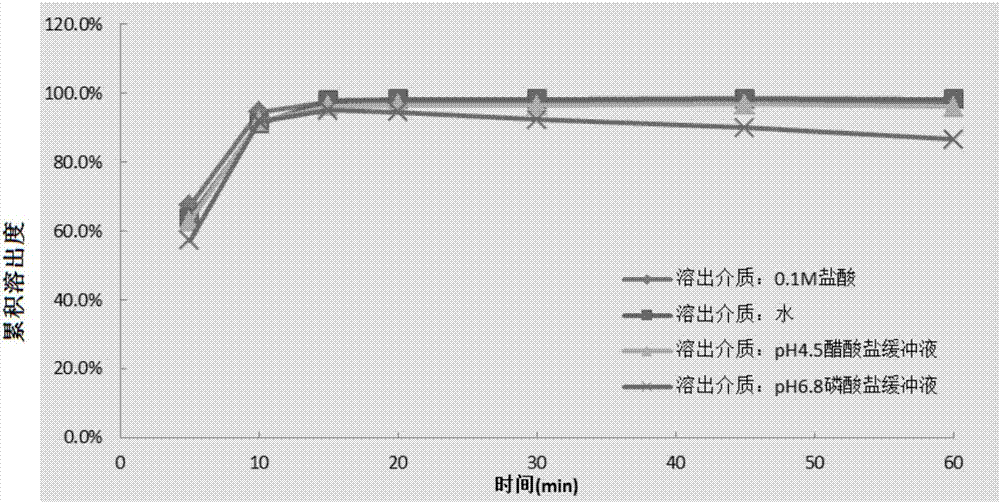
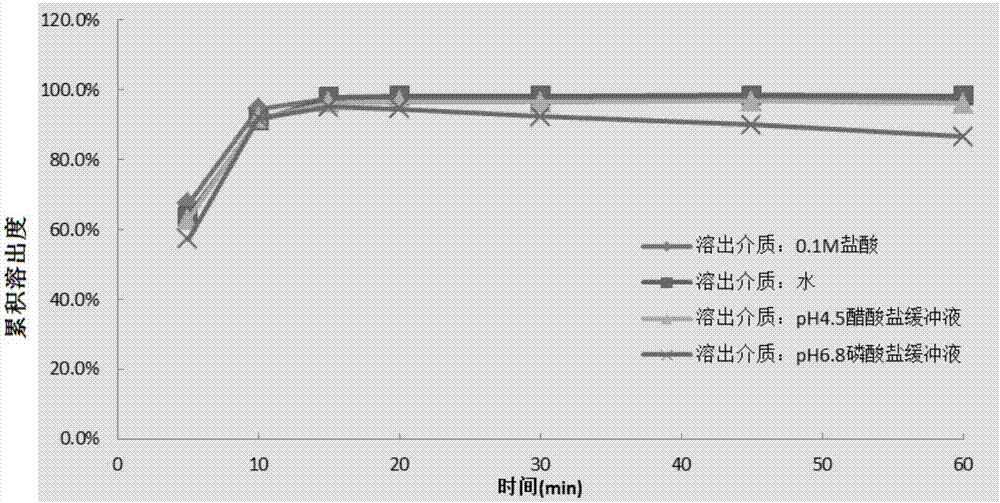
Image
Examples
Embodiment 1
[0048]
[0049]
[0050] In the present embodiment, the formulation of the temozolomide pharmaceutical composition is as follows, and it is made into capsules (an appropriate amount of hollow capsules are used):
[0051] Preparation method: Add 20g of temozolomide, 240g of anhydrous lactose, 15g of croscarmellose sodium, 1g of silicon dioxide, and 5g of tartaric acid into a mixer in sequence, and continue mixing for about 3 minutes. Continue mixing for about 15 minutes after passing the above-mentioned mixed powder through a 20-mesh sieve. Add 5g of glyceryl behenate, continue mixing for about 3 minutes, and fill into 2# hollow capsules, the content of each capsule is about 285mg. The grain weight variation during capsule filling is about ±5%.
Embodiment 2
[0053] In the present embodiment, the formulation of the temozolomide pharmaceutical composition is as follows, and it is made into capsules (an appropriate amount of hollow capsules are used):
[0054] Temozolomide
[0055] Preparation method: Add 100g temozolomide, 205g anhydrous lactose, 25g croscarmellose sodium, 2g silicon dioxide, and 10g tartaric acid into the mixer in sequence, and continue mixing for about 5 minutes. Continue mixing for about 20 minutes after passing the above-mentioned mixed powder through a 24-mesh sieve. Add 8g of glyceryl behenate, continue mixing for about 5 minutes, and fill into 1# empty capsules, the content of each capsule is about 350mg. The grain weight variation during capsule filling is about ±6%.
Embodiment 3
Appropriate amount
[0058] In the present embodiment, the formulation of the temozolomide pharmaceutical composition is as follows, and it is made into capsules (an appropriate amount of hollow capsules are used):
[0059] Preparation method: Add 10g of temozolomide, 180g of anhydrous lactose, 6g of croscarmellose sodium, 1g of silicon dioxide, and 2g of tartaric acid into a mixer in sequence, and continue mixing for about 3 minutes. Continue mixing for about 15 minutes after passing the above-mentioned mixed powder through a 20-mesh sieve. Add 1g of glyceryl behenate, continue mixing for about 5 minutes, and fill into 2# hollow capsules, the content of each capsule is about 200mg. The difference in grain weight during capsule filling is about ±6.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com