Dexmethylphenidate skeleton pattern transdermal patch containing vitiligo inhibitor and preparing method and application thereof
A technology of dexmethylphenidate and transdermal patch is applied in the directions of medical preparations containing active ingredients, medical preparations without active ingredients, nervous system diseases, etc., and can solve the problem of low bioavailability of oral dexmethylphenidate, The environment and human health are unfavorable, and the pharmacological activity of L-body is low, so as to achieve good pharmacological activity and curative effect, avoid the phenomenon of sudden release, and inhibit the formation of vitiligo on the skin.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
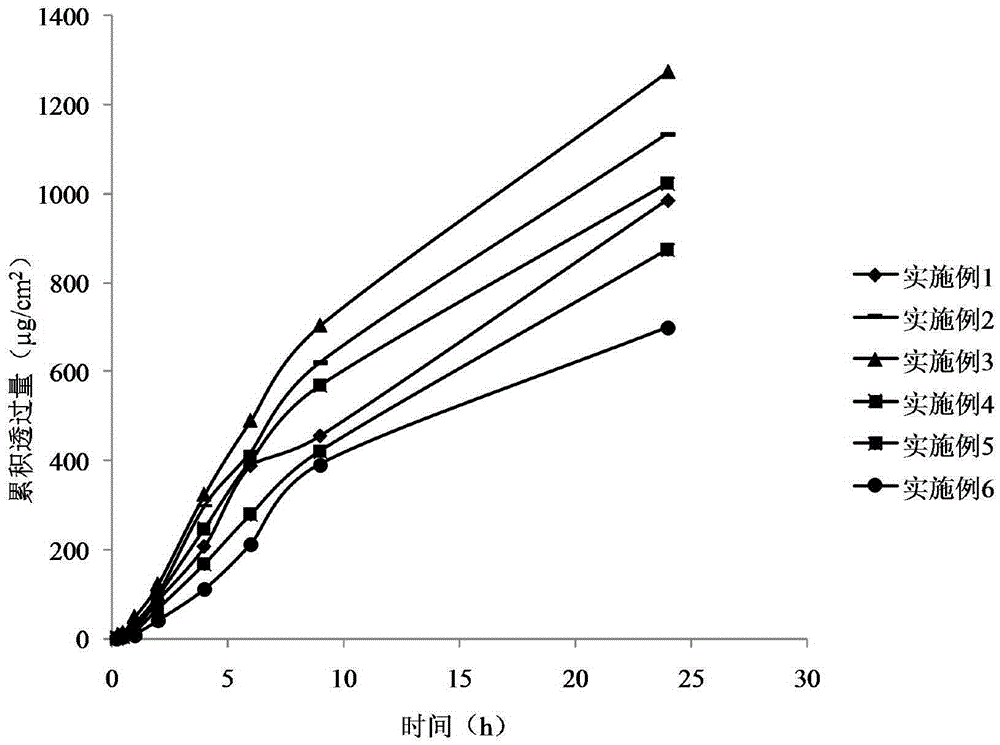
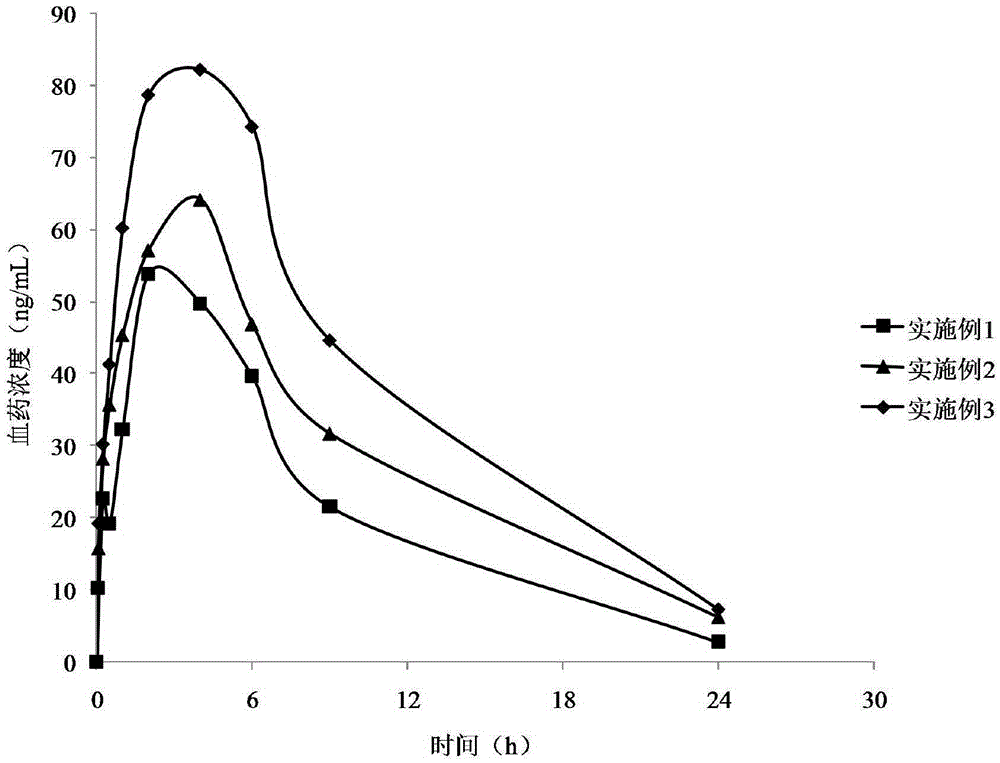
Examples
Embodiment 1
[0045] 【Prescription】(Based on 1000 stickers)
[0046] Dexmethylphenidate Hydrochloride 25g, Dioxidized Peptide 4g, Glycolic Acid 8g, Aminomethyl Propanol 8g, Ethanol 125g, DURO-TAK387-2052 Polyacrylate Pressure Sensitive Adhesive 120g, Ethyl Lauryl 4g, dl-a- Tocopherol 0.5g.
[0047]
[0048] The pressure-sensitive adhesive is a solvent-based pressure-sensitive adhesive, with a weight solid content of 47.5%. After drying, the solvent is removed from the solvent. The total weight is 120g, and it is 57g after drying;
[0049] Preparation:
[0050] Dissolve the dexmethylphenidate hydrochloride, ethyl lauryl alcohol, dl-a-tocopherol of prescription quantity in ethanol, the weight consumption of ethanol is 5 times of dexmethylphenidate hydrochloride; Stir 20 minutes with the speed of 500rpm, obtain clarification Transparent liquid; add dioxidized peptide and glycolic acid to the above liquid, stir at a speed of 1000rpm for 25 minutes to obtain a suspension; add polyacrylate p...
Embodiment 2
[0054] 【Prescription】(Based on 1000 stickers)
[0055] Dexmethylphenidate 18g, tretinoin 10g, ethyl acetate 126g, DURO-TAK87-2287 polyacrylate pressure-sensitive adhesive 180g, isosorbide dimethyl ether 35g, L-ascorbyl palmitate 0.35g.
[0056]
[0057] The pressure-sensitive adhesive is a solvent-based pressure-sensitive adhesive with a solid content of 50.5% by weight. After drying, the solvent is removed from the solvent. The total weight is 180g, and it is 90.9g after drying;
[0058] Preparation:
[0059] Dexmethylphenidate, isosorbide dimethyl ether, L-ascorbyl palmitate of recipe quantity are dissolved in ethyl acetate, and the weight consumption of ethyl acetate is 7 times of dexmethylphenidate; Stir with the speed of 1200rpm 15 Minutes, a clear and transparent liquid was obtained; vitamin A acid was added to the above liquid, and stirred at a speed of 3000rpm for 30 minutes to obtain a clear solution; polyacrylate pressure-sensitive adhesive was added to the suspe...
Embodiment 3
[0063] 【Prescription】(Based on 1000 stickers)
[0064] Dexmethylphenidate 65g, tetrahydromethylpyrimidine carboxylic acid 26g, ethyl acetate 390g, DURO-TAK87-2510 polyacrylate pressure-sensitive adhesive 450g, DowCorningBIO-PSA4202 pressure-sensitive adhesive 100g, 1,2-propylene glycol 50g, 2, 4.7 g of 6-di-tert-butyl-4-methylphenol.
[0065]
[0066] The DURO-TAK87-2510 polyacrylate pressure-sensitive adhesive is a solvent-based pressure-sensitive adhesive, with a weight solid content of 40.5%. After drying, the solvent is removed from the solvent. The total weight and dosage is 450g, and the drying is 182.25g;
[0067] DowCorningBIO-PSA7-4202 pressure-sensitive adhesive is a solvent-based pressure-sensitive adhesive, with a solid content of 60% by weight. After drying, the solvent is removed from the solvent. The total weight and dosage is 100g, and 60g after drying;
[0068] Preparation:
[0069] Dissolve the prescribed amount of dexmethylphenidate, 1,2-propylene glyco...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com