COF film material and preparation method and application thereof
A membrane material and membrane layer technology, which can be used in pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc. The effect of compatibility, good chemical stability, good biocompatibility and chemical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] In this example, a COF membrane is prepared, and the preparation method is as follows:
[0061] (1) Dissolve 10 mg of 2,4,6-triformylphloroglucinol in 30 mL of dichloromethane to form solution a;
[0062] (2) Dissolve 10 mg of p-phenylenediamine hydrochloride in 30 mL of water three times to form solution b;
[0063] (3) Add the aqueous phase solution b obtained in step (2) to the upper layer of the oil phase solution a, and react at 25° C. for 7 hours, and the two monomers are polymerized at the interface of the two phases to form a COF membrane.
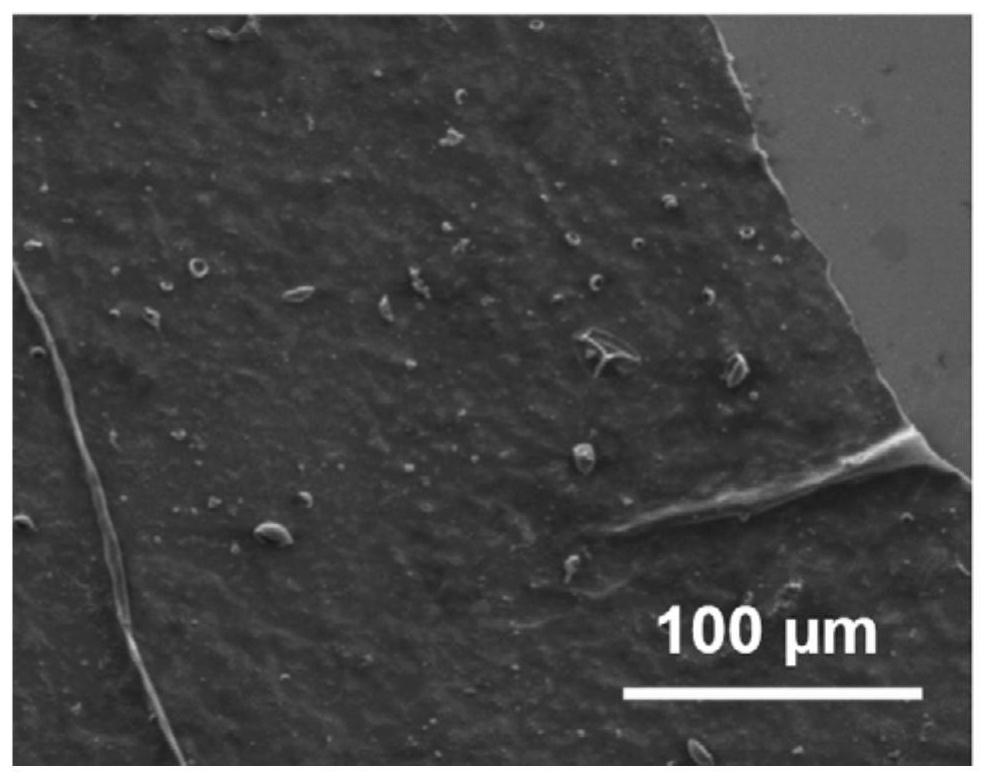
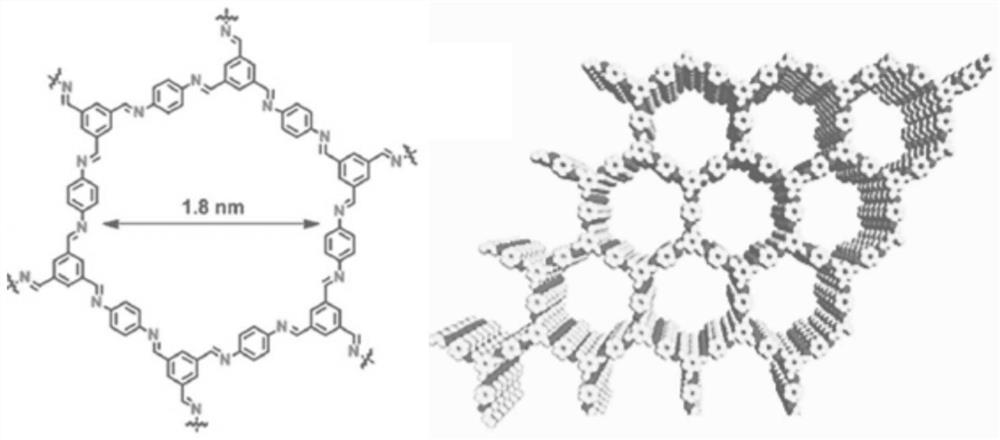
[0064] The prepared COF membrane was observed with a scanning electron microscope, as shown in figure 1 As shown, it can be seen from the figure that the synthesized COF membrane has a large area, is complete and has no defects. The microscopic molecular structure of the COF membrane is as follows: figure 2 shown.
Embodiment 2
[0066] This example prepares a COF membrane material with a sandwich structure, and its preparation method is as follows:
[0067] (1) Spin-coat a solution of polycaprolactone (number-average molecular weight 80,000) with a mass concentration of 500 mg / mL on a silica substrate, spin-coat at a speed of 1500 rpm for 30 seconds, and then spin-coat at a speed of 2000 rpm for 60 seconds to form the first A polycaprolactone layer, dried at 25°C;
[0068] (2) Contact the silicon dioxide base sheet obtained in step (1) with the COF film layer prepared in Example 1, so that the COF film layer is covered on the first polycaprolactone layer, and dried at 25° C.;
[0069] (3) Spin-coat the polycaprolactone solution with a mass concentration of 500mg / mL on the surface of the COF film layer of the product in step (2), spin-coat at a speed of 1500rpm for 30s, and then spin-coat at a speed of 2000rpm for 60s to form a second Polycaprolactone layer, dried at 25°C;
[0070] (4) Etching the si...
Embodiment 3
[0072] In this example, the COF membrane material prepared in Example 2 is tested for the release performance of the drug molecule oxytocin, and the operation method is as follows:
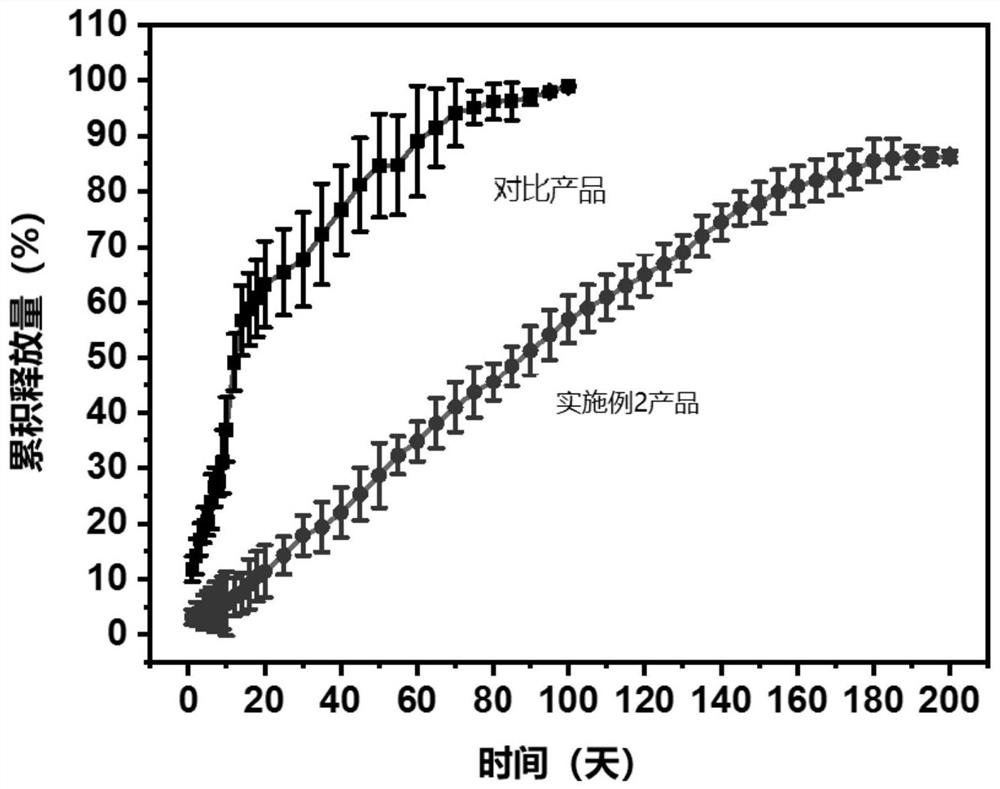
[0073] Oxytocin was placed in the drug release test device, the COF membrane material was placed on top and sealed, and the entire device was placed in phosphate buffer at 37°C to simulate physiological conditions, and the oxytocin (ELISA) kit was used to determine the phosphate buffer The content of oxytocin released in the medium, and calculate the cumulative release amount; at the same time, use the product prepared by the following method as a comparison, and use the same test method to calculate the cumulative release amount, such as image 3 shown by image 3 It can be seen that simple polycaprolactone film cannot obtain zero-order sustained-release behavior of oxytocin, but the COF film material involved in the present invention can obtain zero-order sustained-release behavior of oxytocin a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com