Medication risk assessment system
A risk assessment system and risk technology, applied in the field of medical diagnosis and treatment system design, can solve problems such as irregular disease treatment, shortage of medical resources, and weak awareness of patients' rational use of drugs, and achieve the effect of intuitively prompting systemic risks.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0020] In order to explain in detail the technical content, structural features, achieved goals and effects of the technical solution, the following will be described in detail in conjunction with specific embodiments and accompanying drawings.
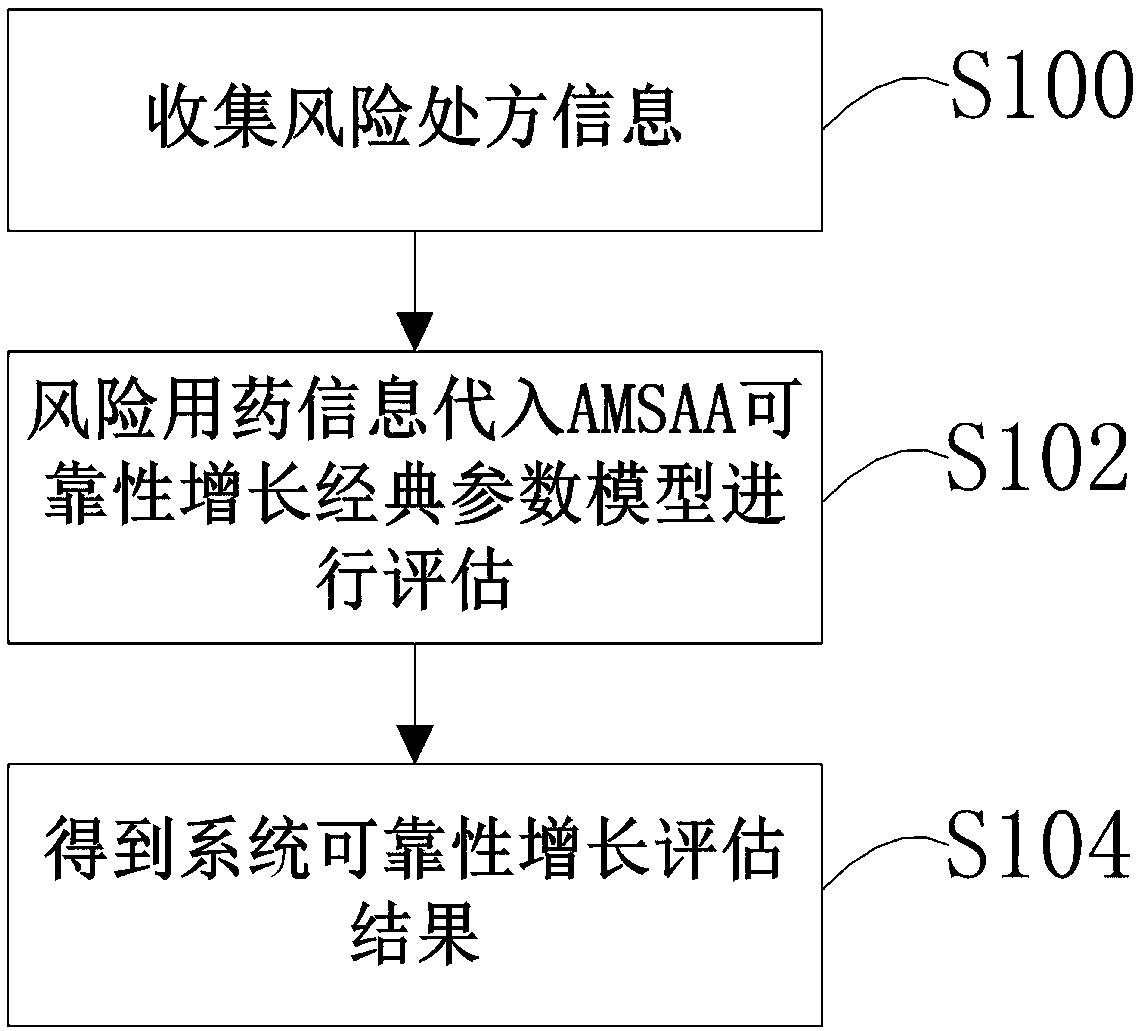
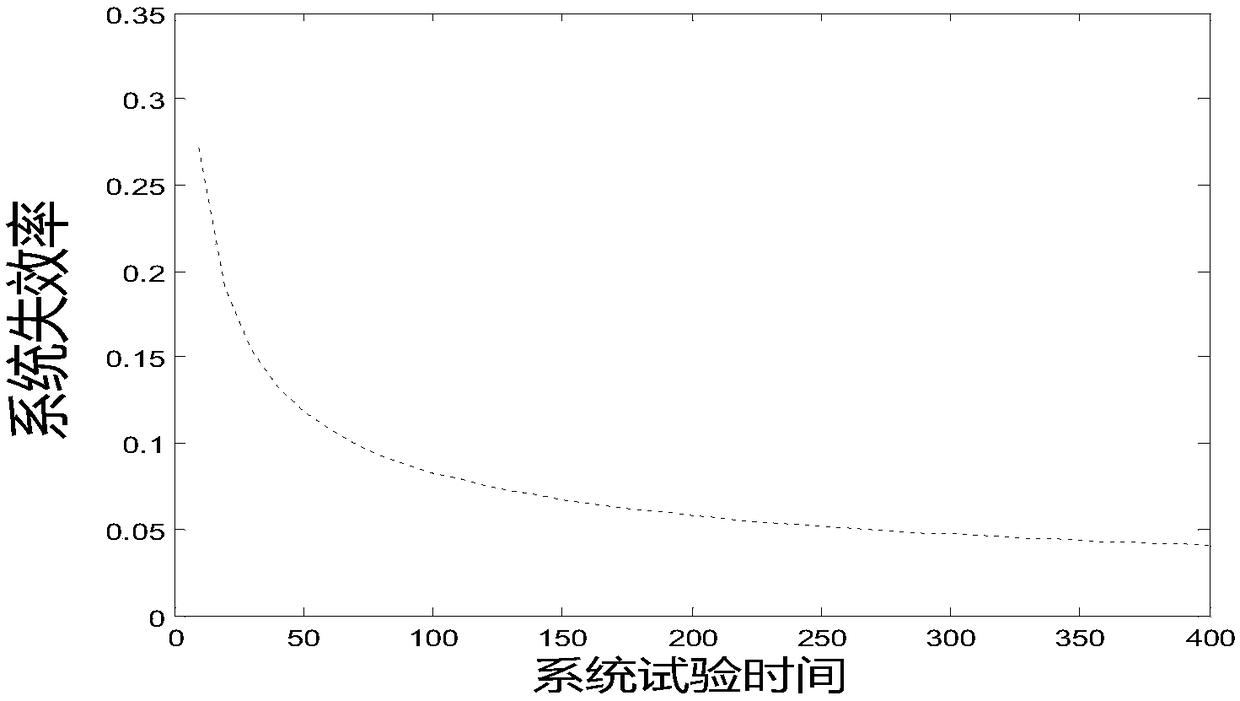
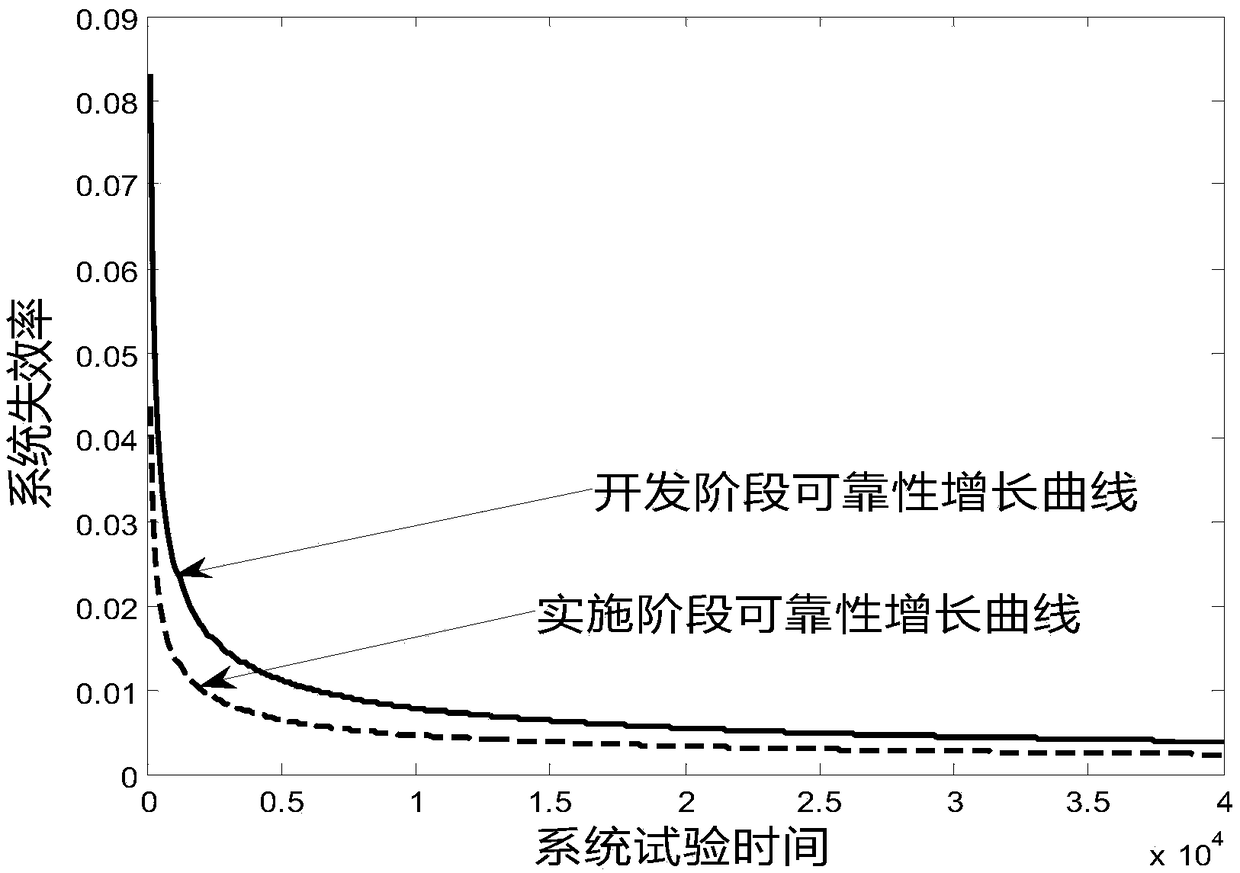
[0021] see figure 1 , is a medication risk assessment method in the present invention. This embodiment includes the following steps. S100 collects risk prescription information, and the risk medication information includes risk prescription information, risk accumulation time information, and risk medication times. S102 substitutes risk medication information into The classic parameter model of AMSAA reliability growth is evaluated, and S104 obtains the evaluation result of system reliability growth. In our example, medication risk is an objective situation assessment relative to human-operated medication conditions. Therefore, if there are scientific means to conduct stable and objective assessment, it will help improve the treatment...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com