Active monitoring and early warning system for drug-induced diseases
A technology for monitoring, early warning, and disease, applied in the medical field, can solve problems such as missed opportunities for ADE intervention, false positives in the ADE/ME monitoring system, poor timeliness of samples, etc., to achieve the effect of ensuring accuracy and strengthening risk management and control of clinical medication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0129] Example 1: For patients over 65 years old with a history of falls or fractures, the drugs on the high-risk drug list include: midazolam, diazepam, alprazolam, diazepam, nitrazepam, and clonazepam , lorazepam, sulpiride, flupenthixol, melitracen, risperidone, clozapine, droperidol.
[0130] Drugs in the list of contraindicated drugs include: sulpiride, flupenthixol, melitracen, risperidone, clozapine, droperidol. The above list can be sent to the patient's physician in charge to remind the risks of medication.
example 2
[0131] Example 2: When the patient in Example 1 has new symptoms of heart failure, the newly added drugs in the prohibited drug list for the patient should include: cilostazol and non-steroidal anti-inflammatory drugs.
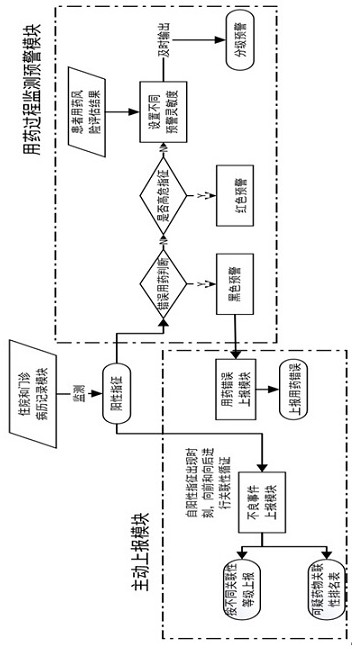
[0132] Compared with the prior art, the beneficial effect of the present invention is that the patient medication risk prompt module can output the latest prohibited drug list and high-risk medication list based on the patient's real-time physical condition, and send them to the competent physician and pharmacist in the form of a list of precautions for medication. Clinical medication provides accurate and timely reference, forming the first level of risk prevention and control beforehand.
[0133] The patient medication risk assessment module comprehensively evaluates the possibility of adverse drug events occurring in the patient according to the patient's physical condition and medication situation.
[0134] Preferably, the patient medication risk assessmen...
example 3
[0151] Example 3: Adverse events in the adverse event set of ibuprofen include: liver injury, parameters of adverse events related to liver injury include: (1) ALT>40U / L (upper limit of normal value), TB>1×ULN ( Upper limit of normal value), (2) ALT>2×ULN (2 times upper limit of normal value) in a single test, TB>2×ULN (upper limit of normal value). The ADE / ME intervention parameters of ibuprofen-induced liver injury include: (1) discontinuation or reduction of ibuprofen, (2) glutathione, tiopronin, glycyrrhizic acid preparations, polyene phosphorylcholine apply.
[0152] The medication process monitoring and early warning module includes: (1) a graded early warning sub-module, which is used to output different levels of monitoring of suspected adverse drug events, upcoming medication errors, and medication errors that have occurred according to risk levels. early warning. (2) Risk degree monitoring and early warning sub-module, which is used to: monitor the patient treatmen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com