Active monitoring and reporting system for drug-induced diseases
A disease and drug source technology, applied in the medical field, can solve problems such as missed ADE intervention opportunities, ADE/ME monitoring system false positives, small sample size of original research data, etc., to achieve the effect of ensuring accuracy and strengthening clinical drug risk management and control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
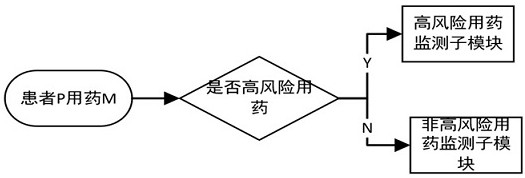
[0112] Example 1: For patients over 65 years old with a history of falls or fractures, the drugs on the high-risk drug list include: midazolam, diazepam, alprazolam, diazepam, nitrazepam, and clonazepam , lorazepam, sulpiride, flupenthixol, melitracen, risperidone, clozapine, droperidol.
[0113] Drugs in the list of contraindicated drugs include: sulpiride, flupenthixol, melitracen, risperidone, clozapine, droperidol. The above list can be sent to the patient's physician in charge to remind the risks of medication.
example 2
[0114] Example 2: When the patient in Example 1 has new symptoms of heart failure, the newly added drugs in the banned drug list for the patient should include: cilostazol and non-steroidal anti-inflammatory drugs.
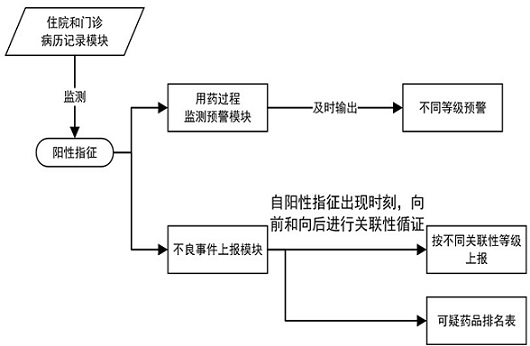
[0115] Compared with the prior art, the beneficial effect of the present invention is that the patient medication risk prompt module can output the latest prohibited drug list and high-risk medication list based on the patient's real-time physical condition, and send them to the competent physician and pharmacist in the form of a list of precautions for medication. Clinical medication provides accurate and timely reference, forming the first level of risk prevention and control beforehand.
[0116] The medication error monitoring module is used to judge whether the patient's current medication is wrong. Preferably, for the medicine currently used by the patient, it is judged whether the medicine belongs to the contraindicated medicine of the patient according to t...
example 3
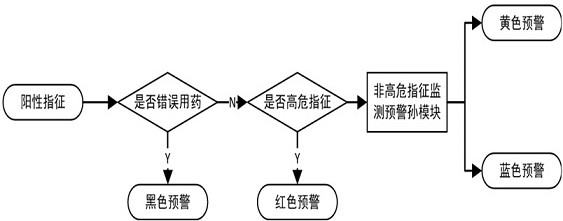
[0119] Example 3: Adverse events in the adverse event set of ibuprofen include: liver injury, parameters of adverse events related to liver injury include: (1) ALT>40U / L (upper limit of normal value), TB>1×ULN ( Upper limit of normal value), (2) ALT>2×ULN (2 times upper limit of normal value) in a single test, TB>2×ULN (upper limit of normal value). The ADE / ME intervention parameters of ibuprofen-induced liver injury include: (1) discontinuation or reduction of ibuprofen, (2) glutathione, tiopronin, glycyrrhizic acid preparations, polyene phosphorylcholine apply.
[0120] The medication process monitoring and early warning module includes: (1) hierarchical early warning sub-module, which is used to output different levels of early warnings for monitored suspected adverse drug events, upcoming medication errors, and medication errors that have occurred. (2) The doctor's order medication monitoring sub-module, which is used to check the medication errors when the doctor's order...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com