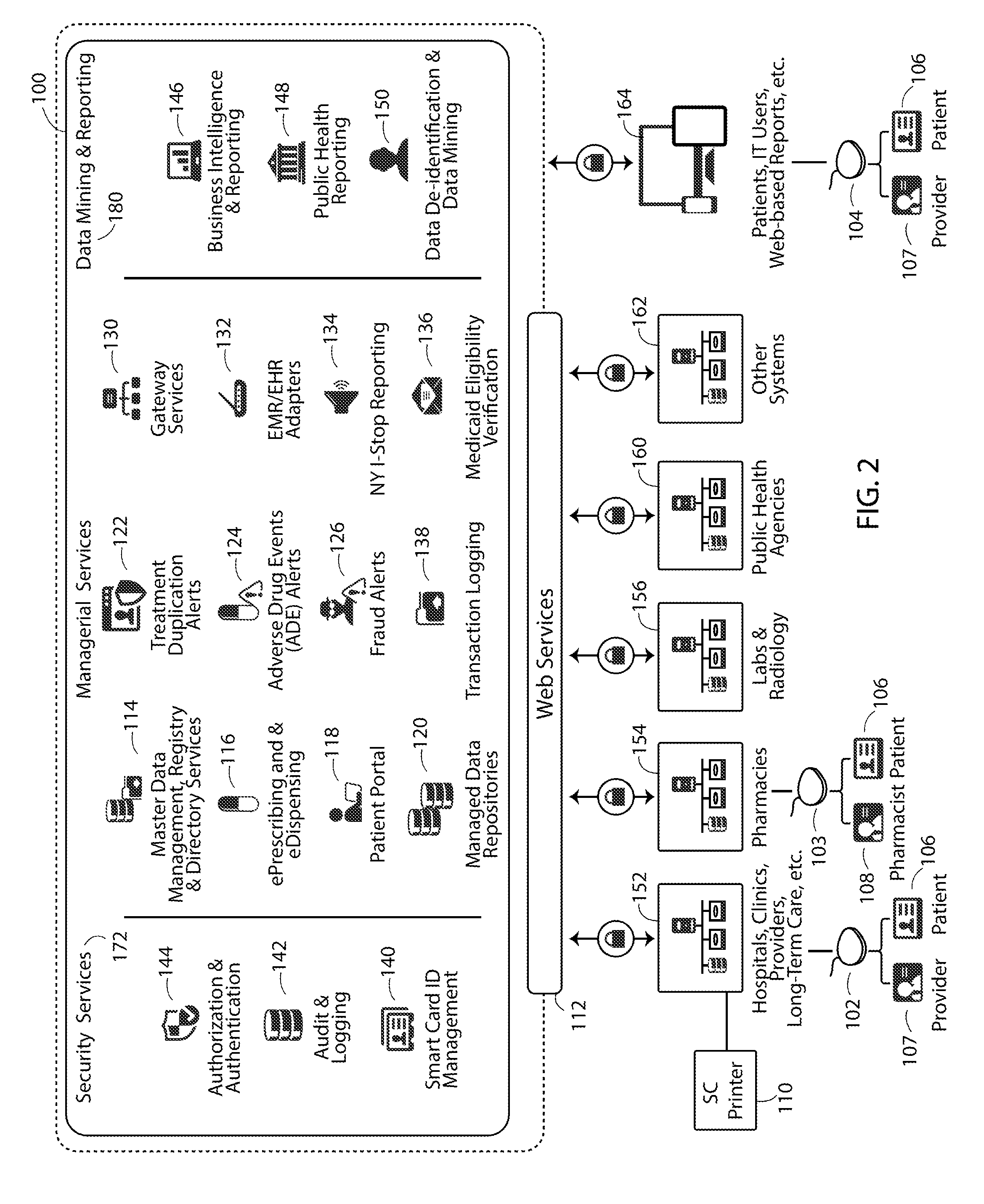
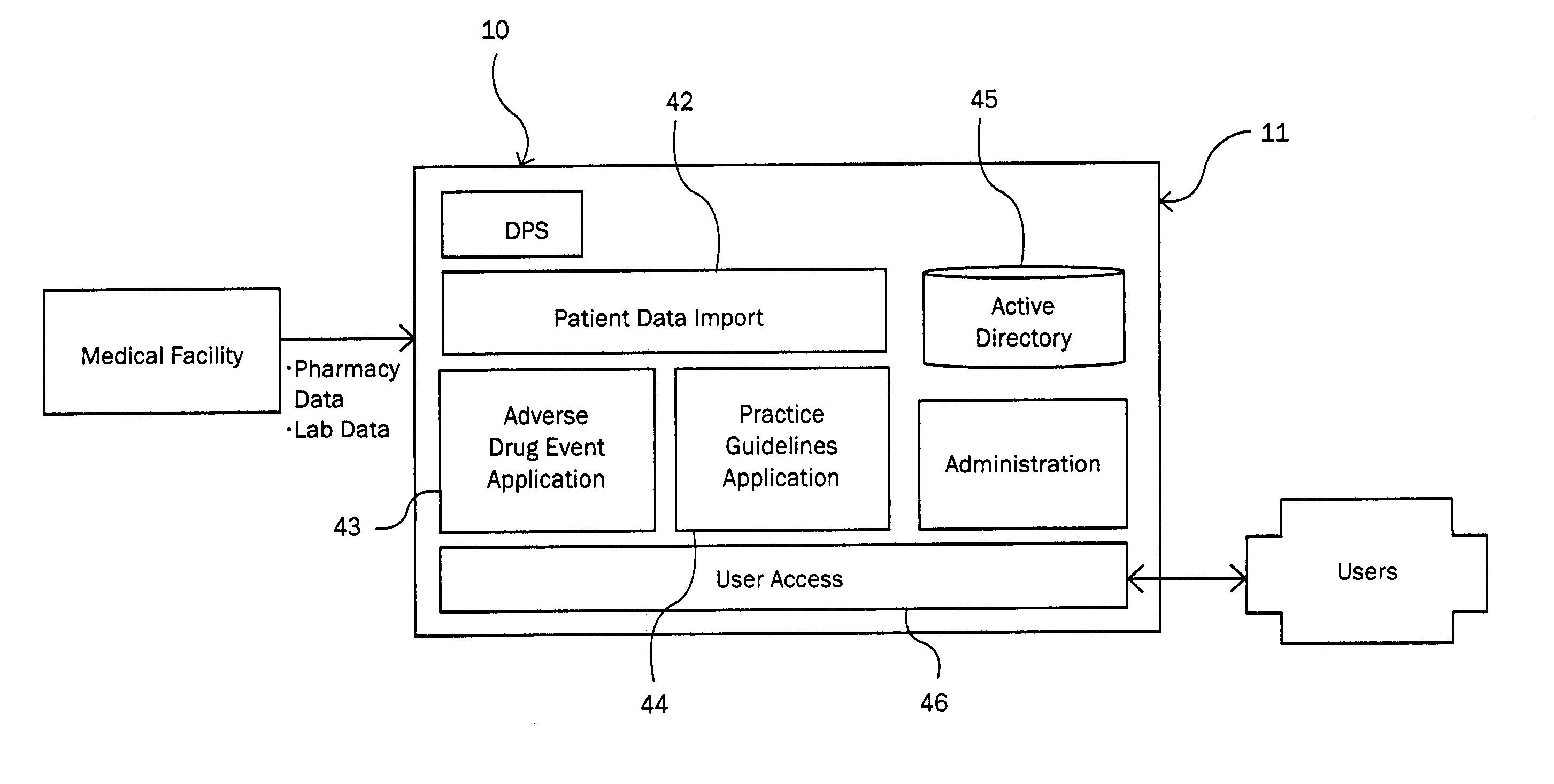
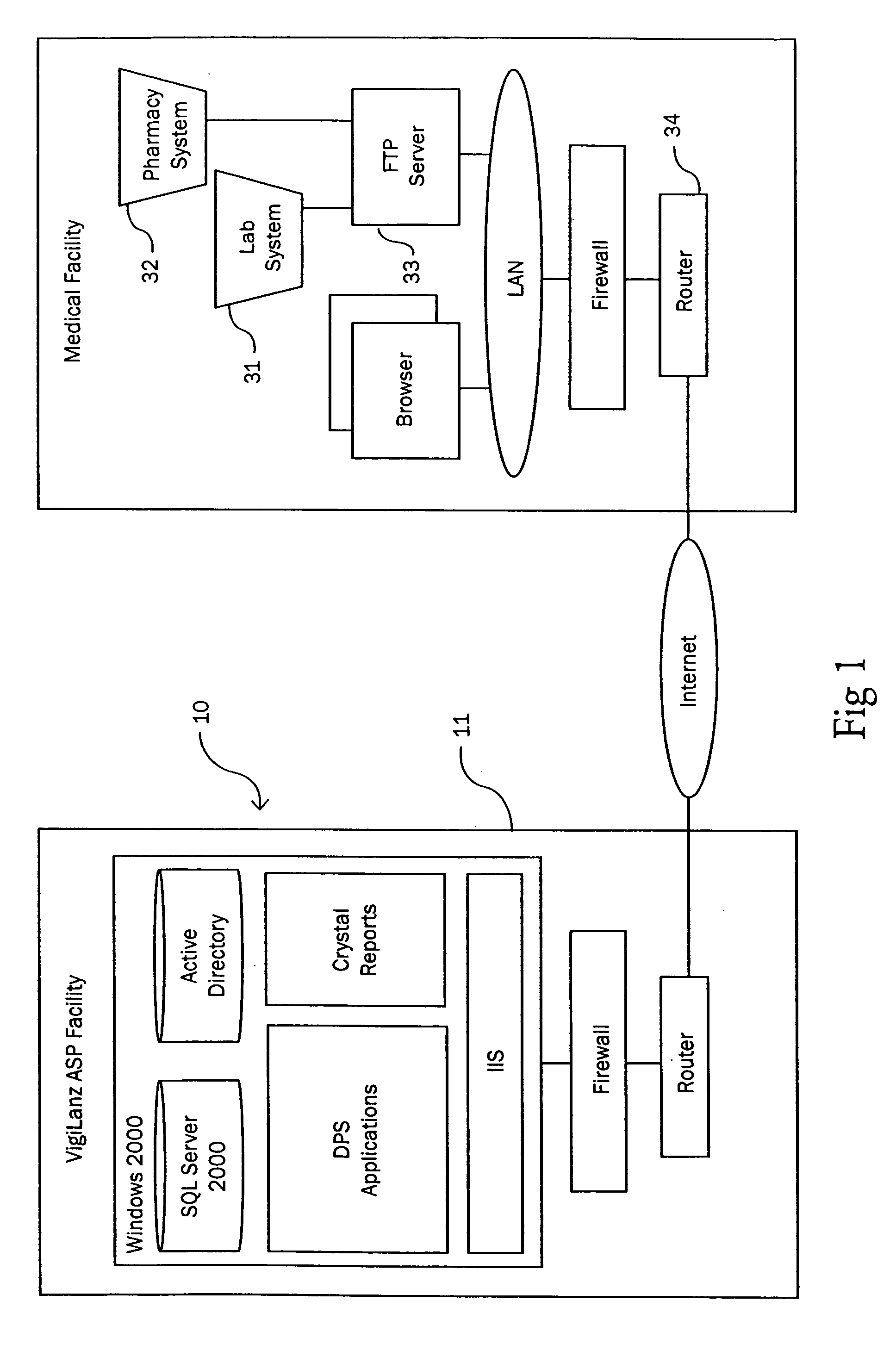
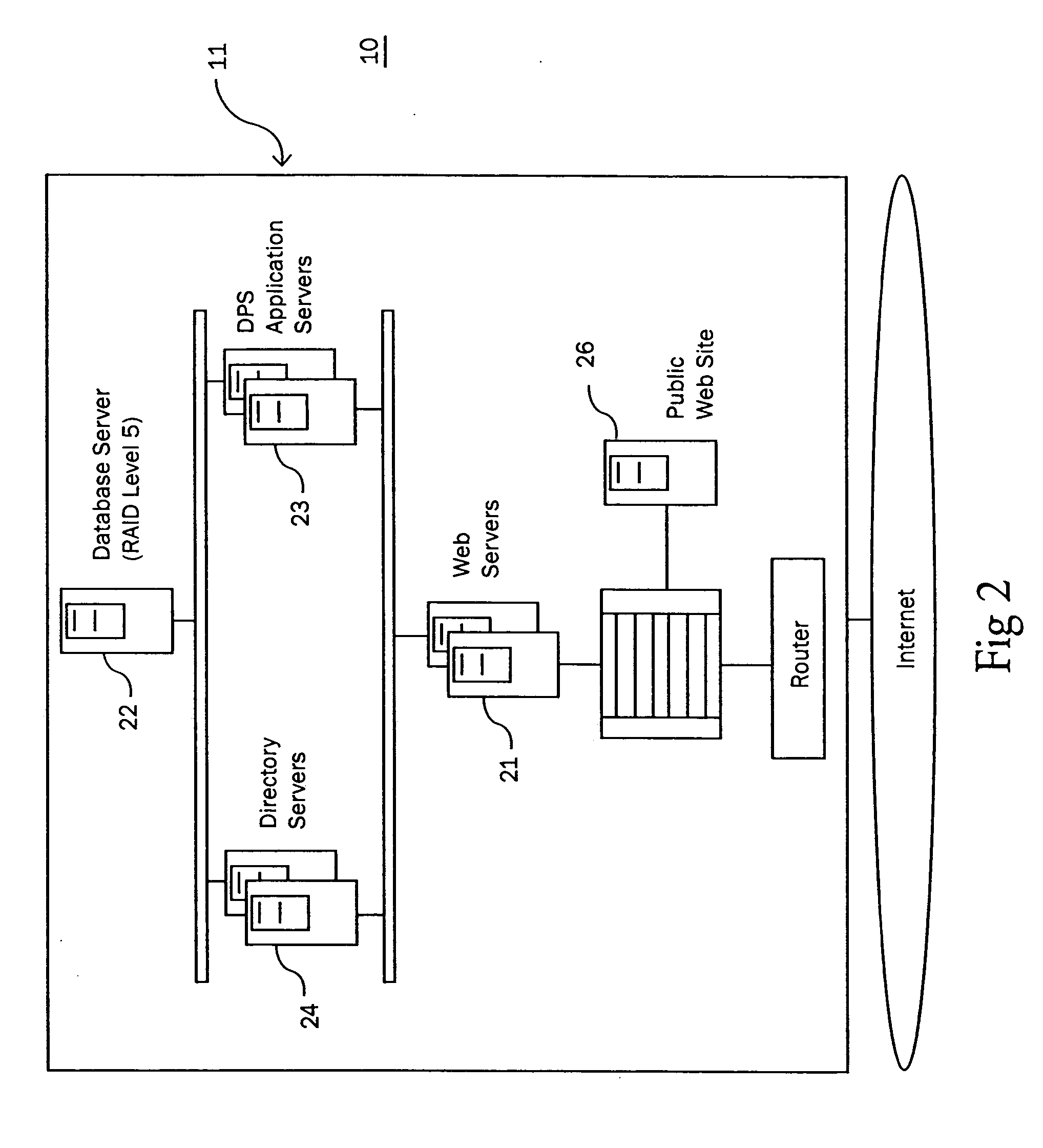
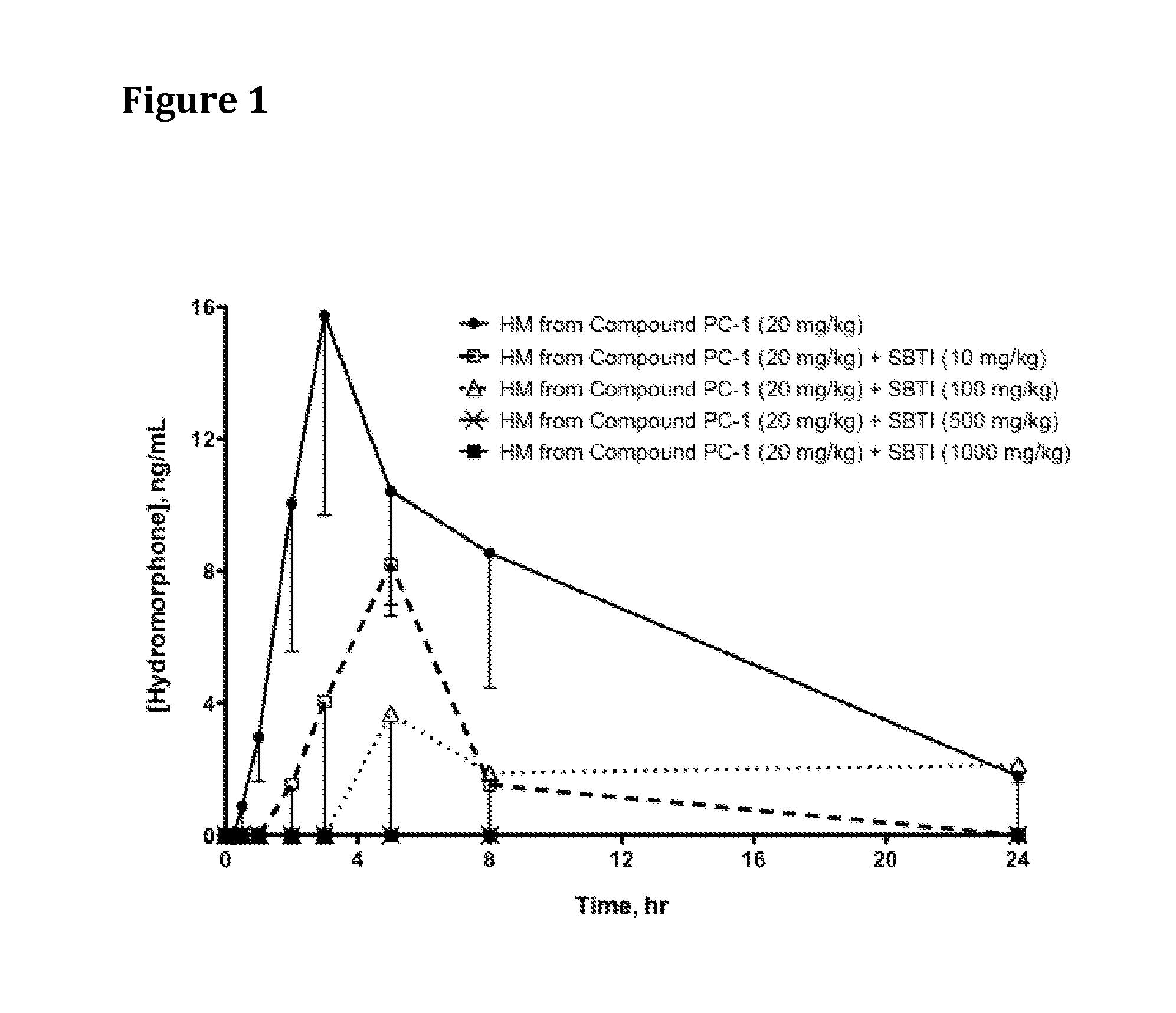
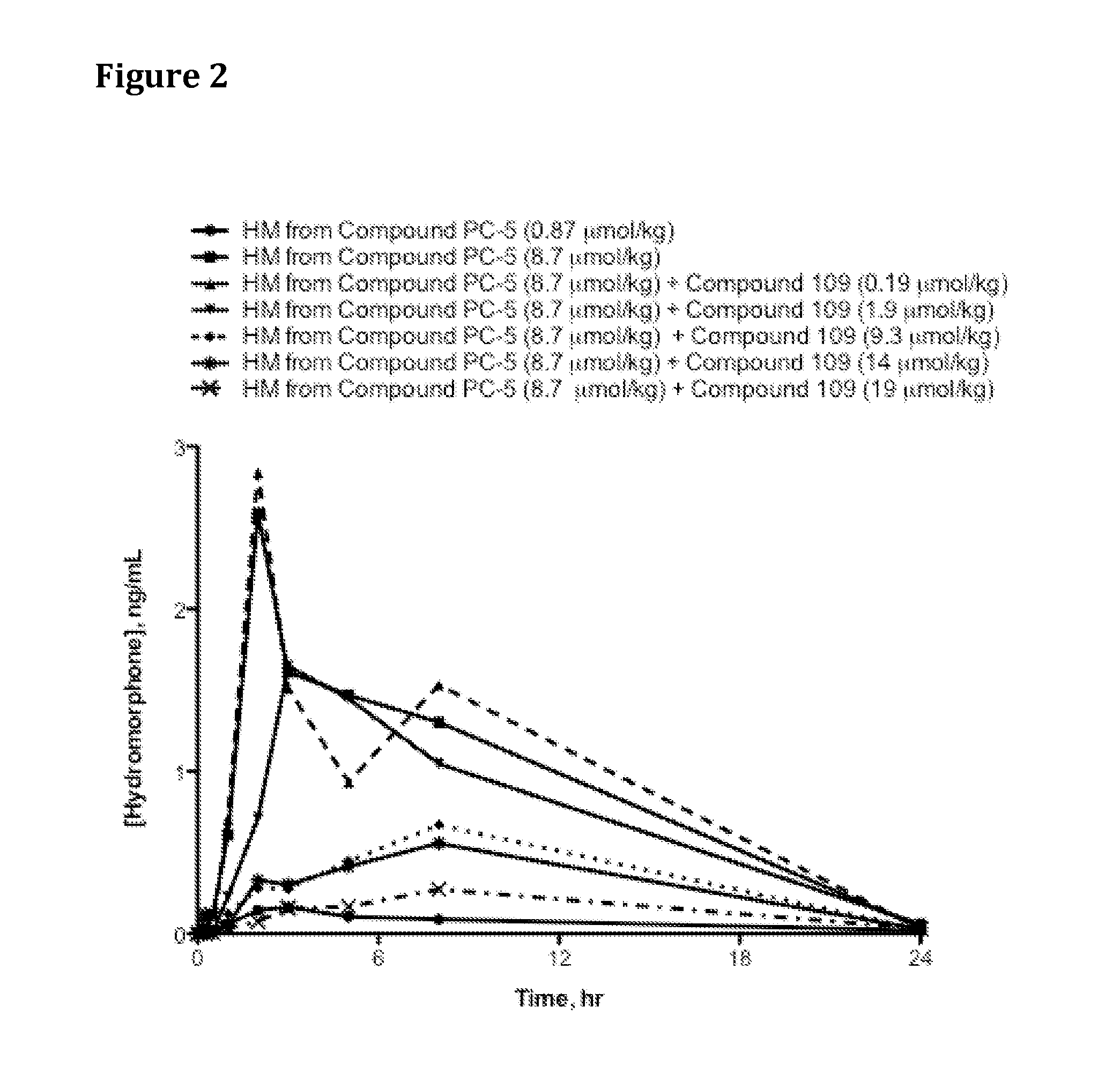
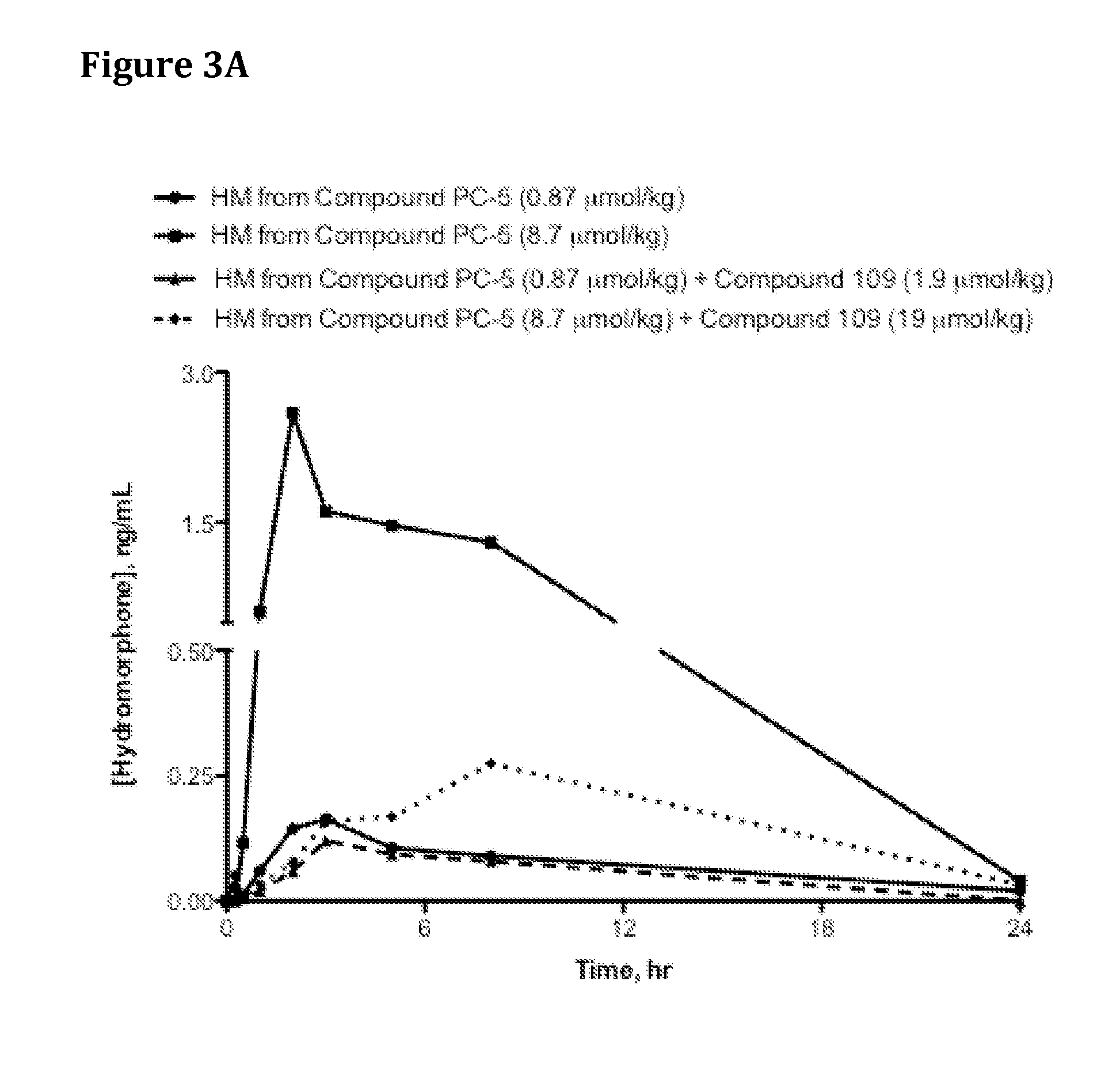
Patents
Literature
32 results about "Adverse drug event" patented technology
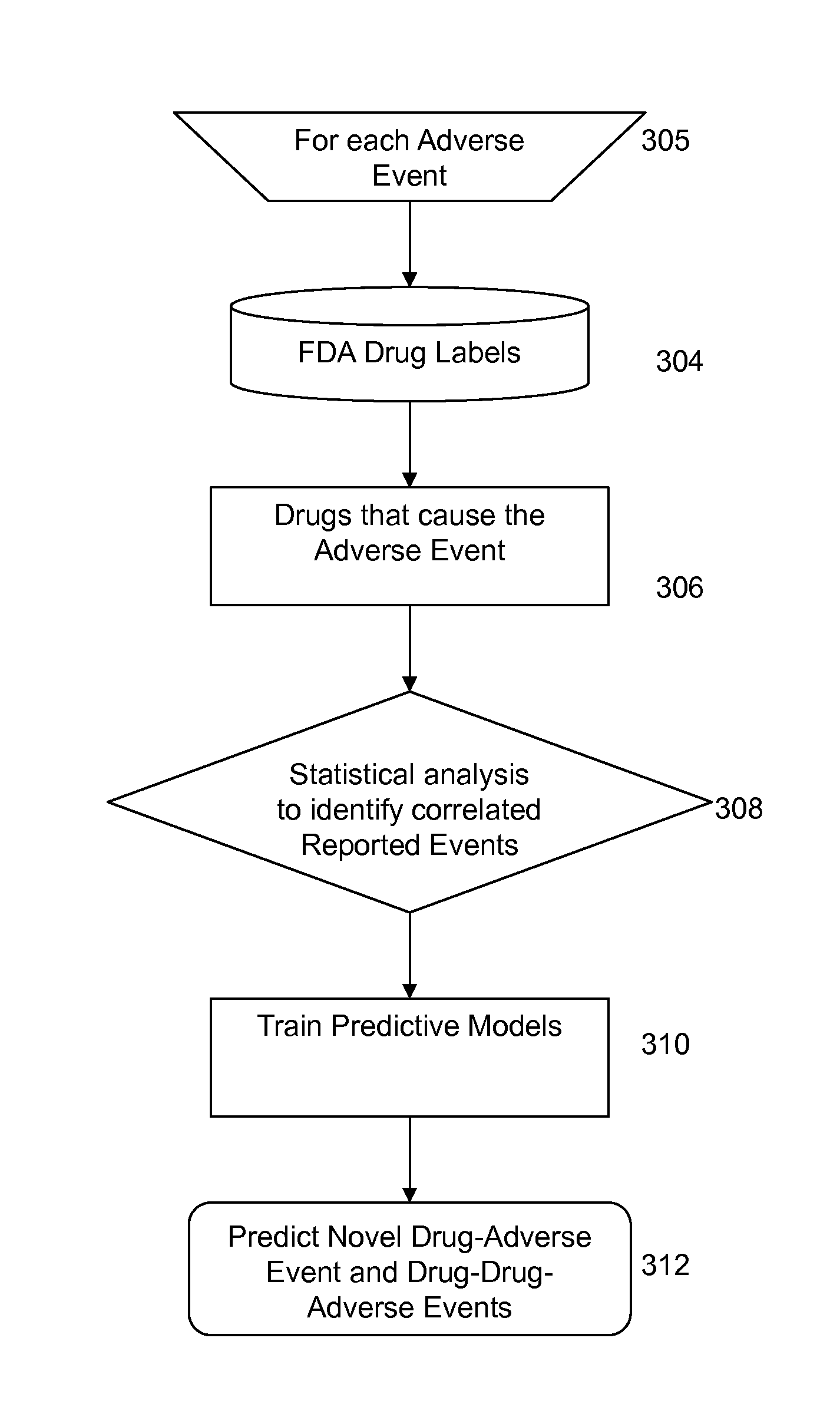
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An adverse drug event is when someone is harmed by a medicine. Certain types of adverse drug events are more common for specific medication classes, such as low blood sugar (hypoglycemia) related to insulin use.
Smart medical compliance method and system
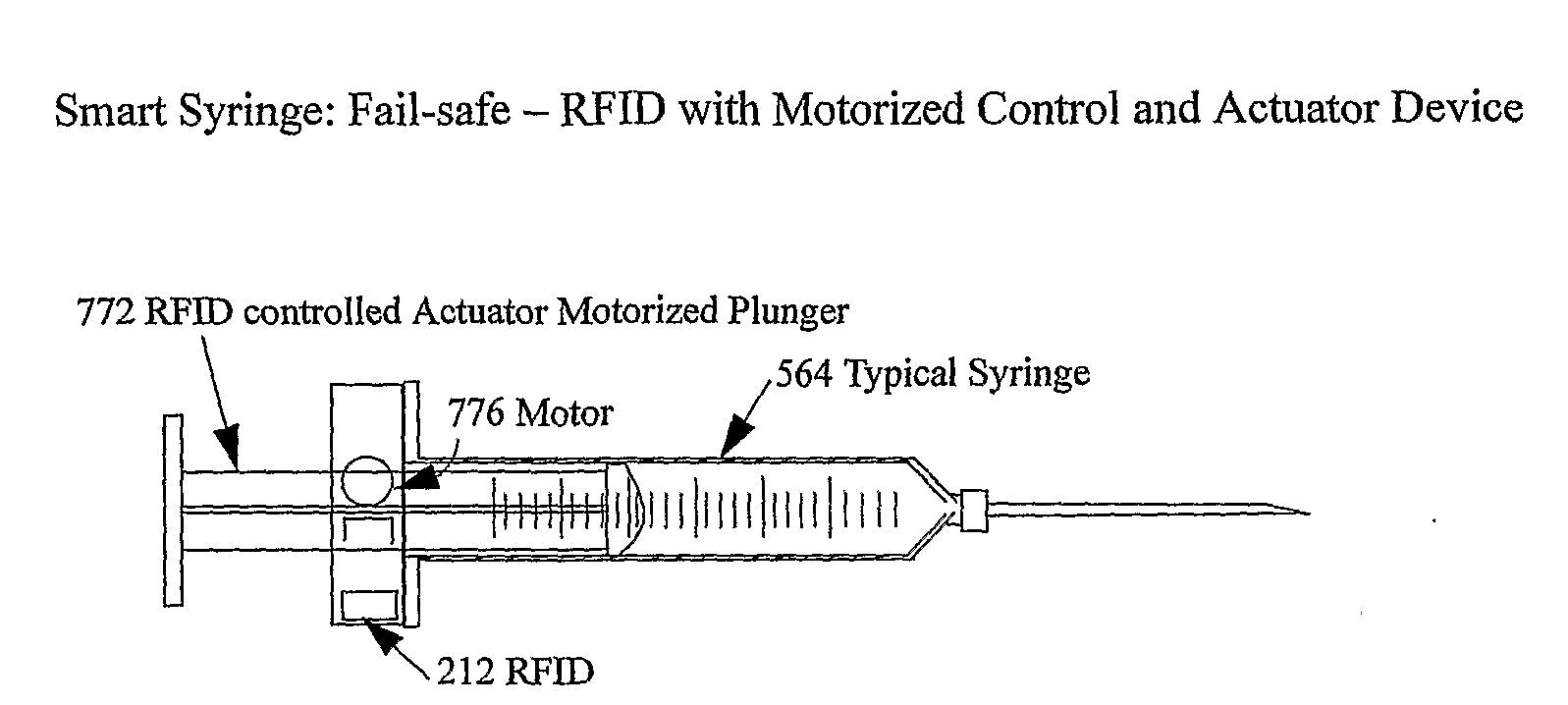
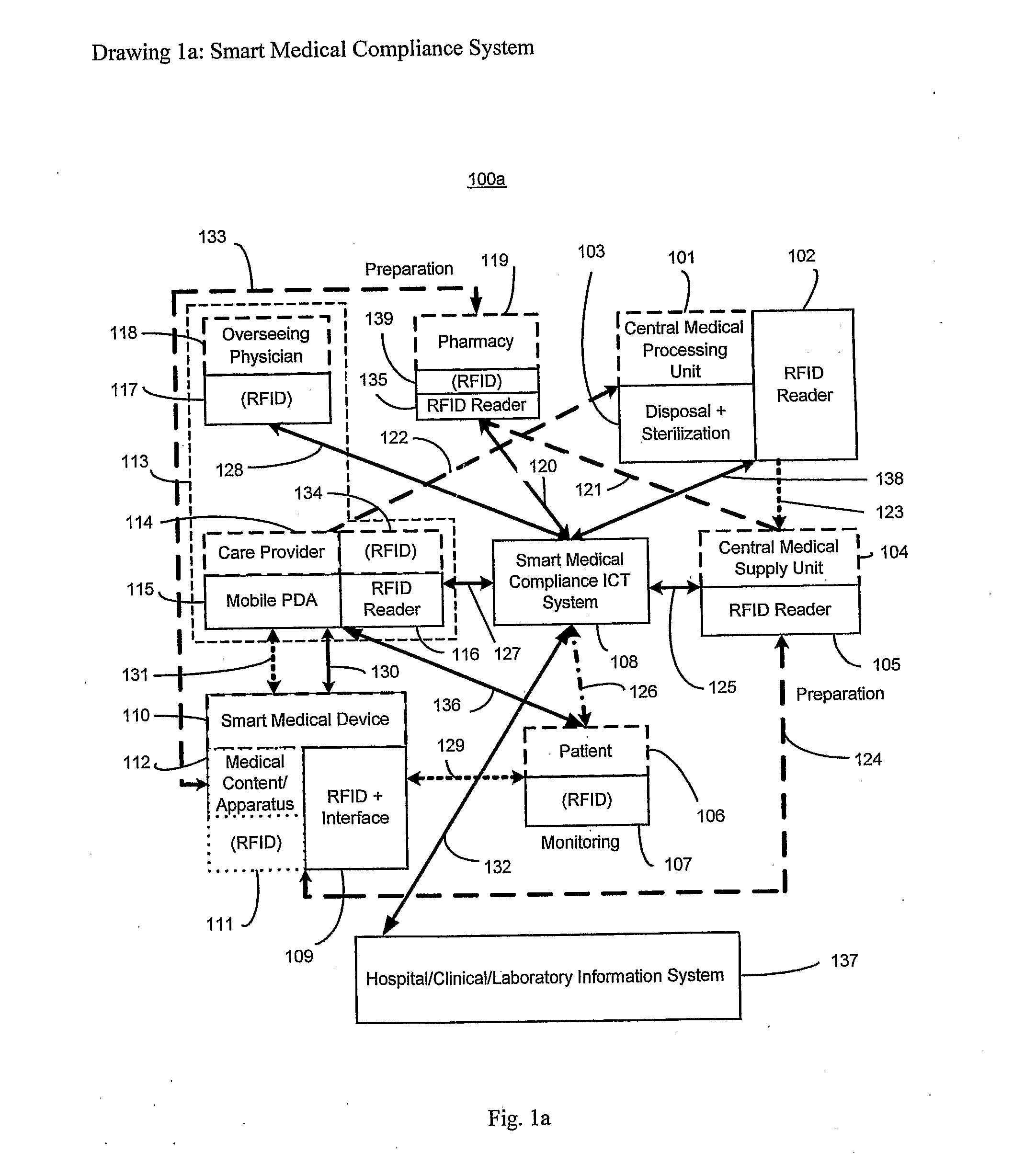
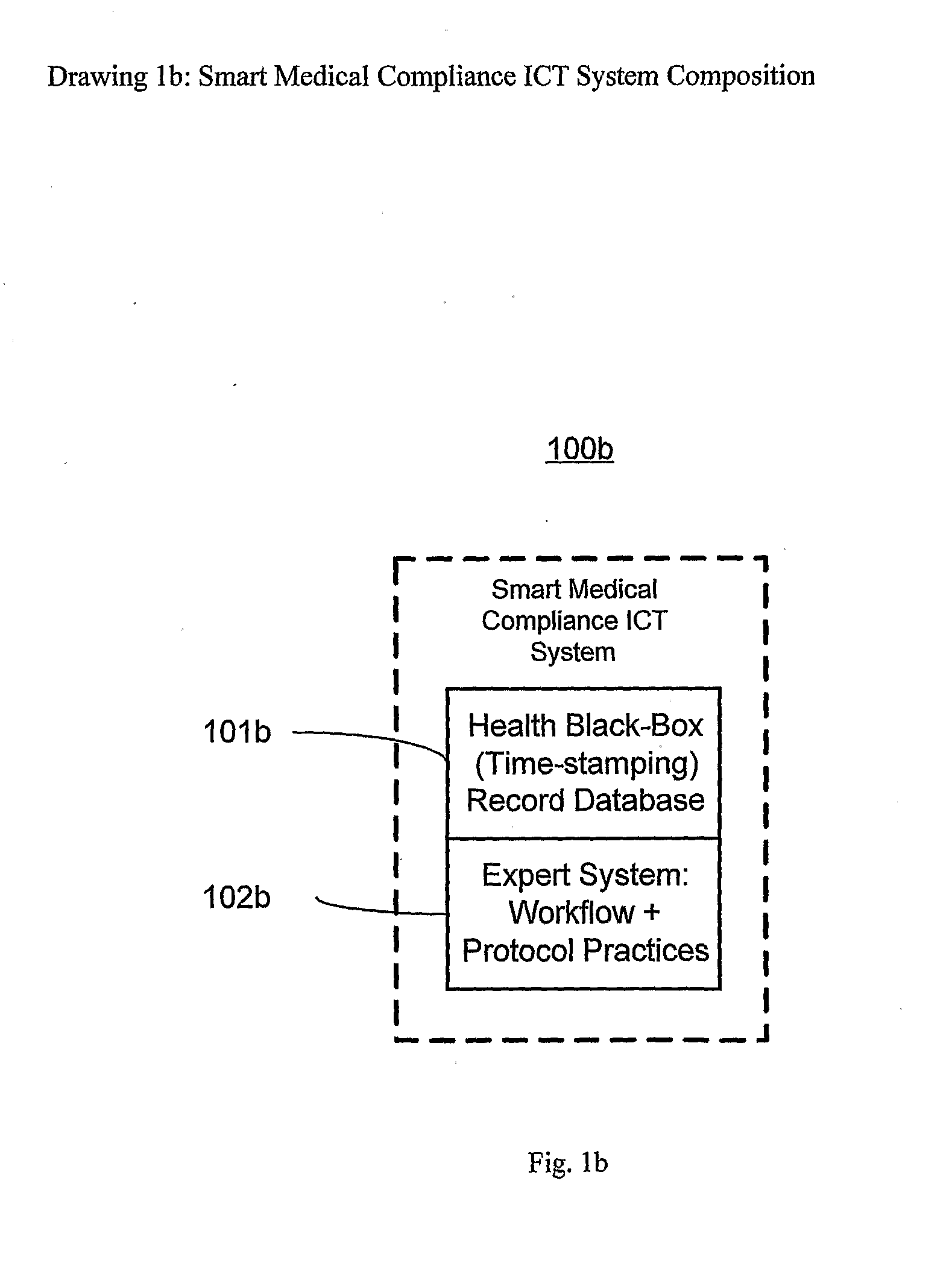
The smart medical compliance method and system invention prevents adverse drug events through the use of protocols that uniquely identifies the patient, care provider, medication and / or medical device that is to be used with radio frequency identification (RFID). The RFID devices incorporate fail-safe locks or indicators that prevent the inadvertent or unauthorized use of medication, medical devices, or medical supplies. The system corroborates, patient, the care provider, the medical device, and the manner in which it is to be used, and authorizes the action to be undertaken through an interface on a personal digital assistant PDA over a wireless communication channel. The system also timestamps events in the equivalent of a medical black box such that records may be kept to further improve patient care and allow an analysis of procedures. In addition, the system includes interfaces to medication preparation and safe disposal. A number of smart devices that interact with the system are also described. These include smart medical containers, smart clamps, smart valves, smart syringes, smart couplers, smart pipettes, and a host of other point of care devices.
Owner:KYAB LULEA
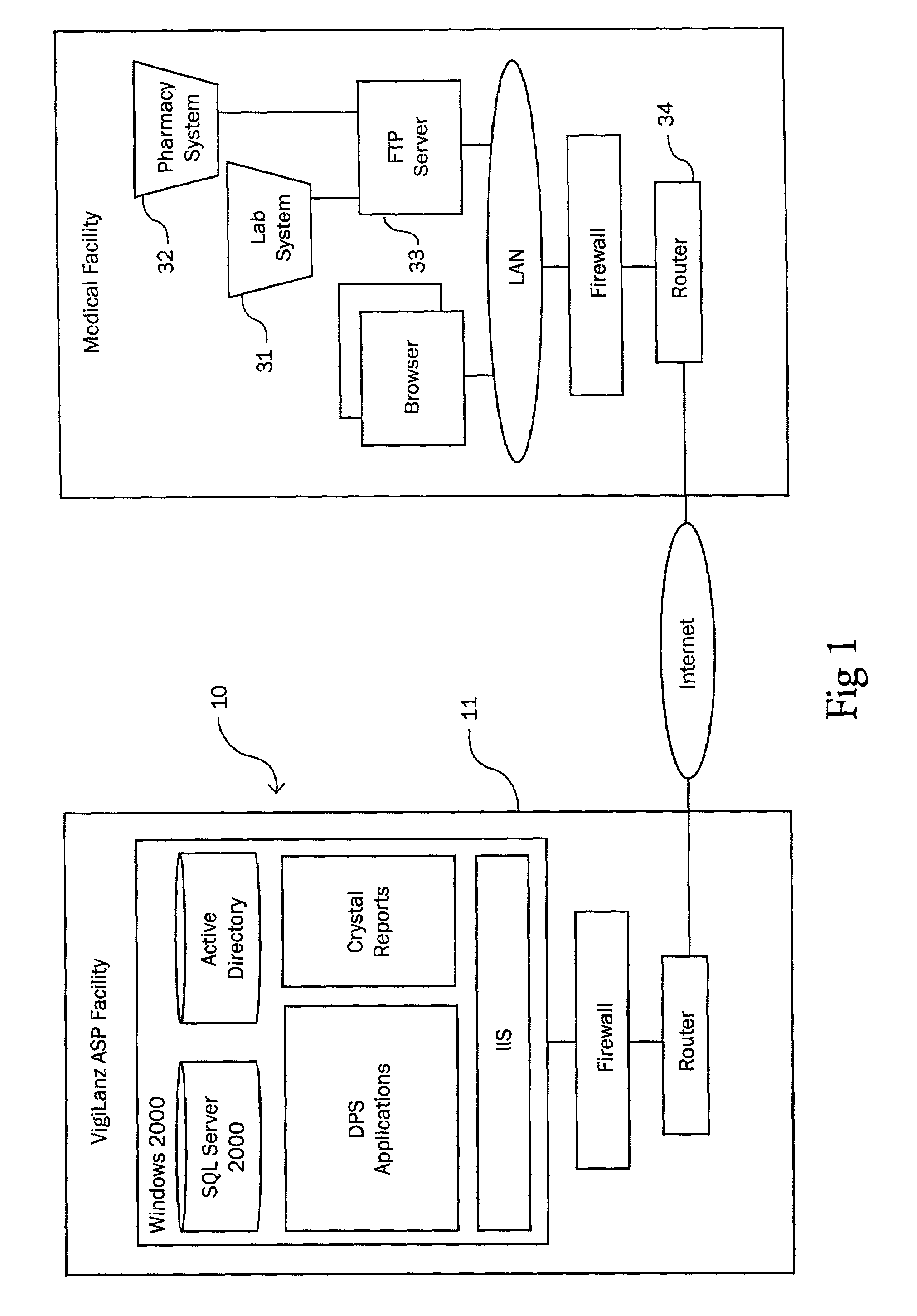
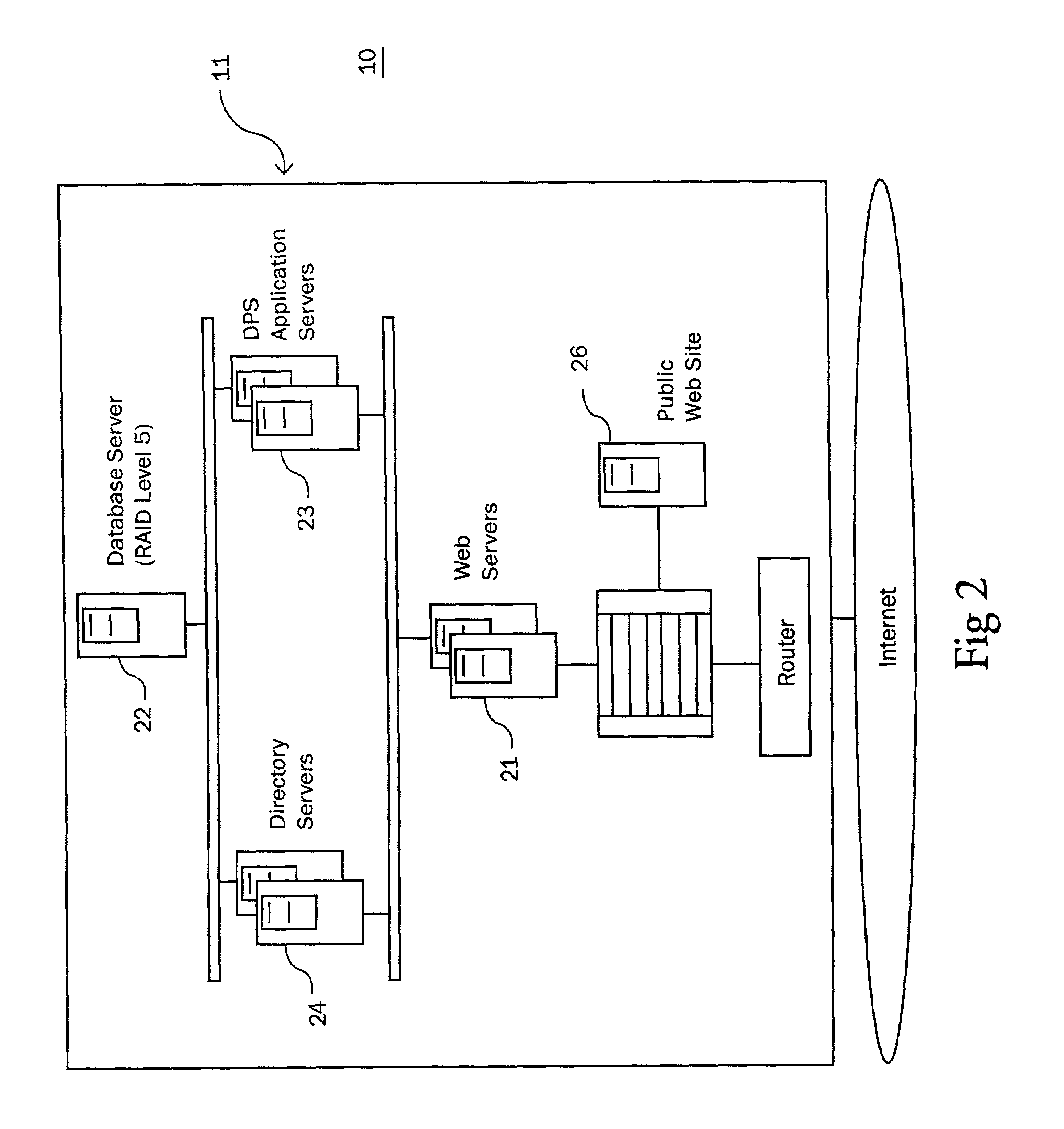
Method and system for identifying and anticipating adverse drug events
InactiveUS6993402B2Drug and medicationsComputer-assisted medicine prescription/deliveryPharmacyTechnical standard
The present invention is a system and method for anticipating potential Adverse Drug Events (ADE) in a patient's medication regimen by integrating data typically located in laboratory and pharmacy information systems and filtering the data using predefined criteria. The present invention includes a system for anticipating a possible ADE through the use of a search engine that compares integrated data from laboratory and pharmacy information systems and compares it to predefined ADE rules defining normal ranges for a particular laboratory test. If an abnormal test value is received and a drug in the patient's medication regimen satisfies a drug included in an ADE rule then an alert procedure is triggered which allows for a period of time wherein the patient's lab and pharmacy data is monitored in order to determine if a proper corrective action is undertaken, and if no corrective action or an improper corrective action is taken within that period of time, the healthcare provider is warned of a potential ADE.
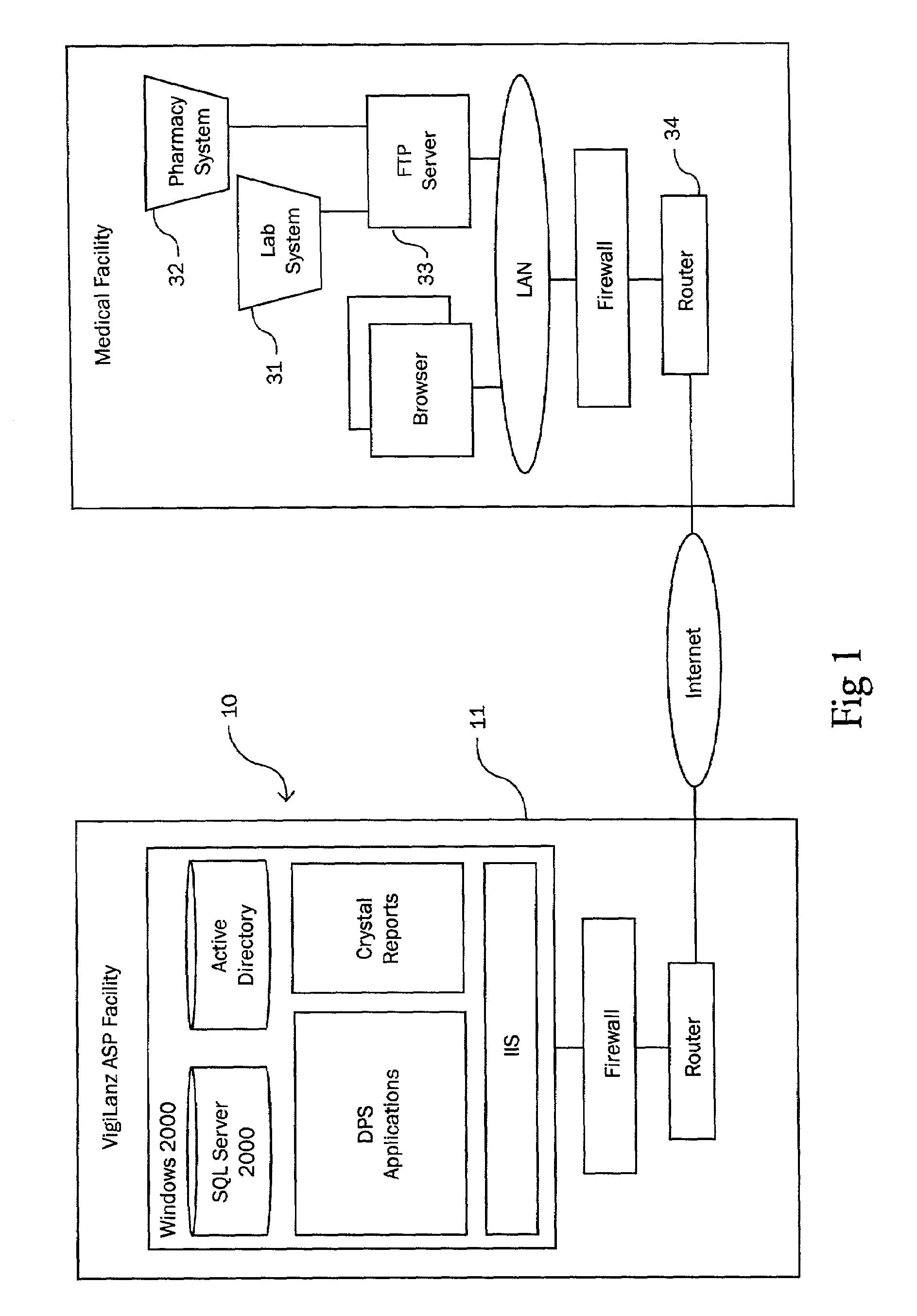
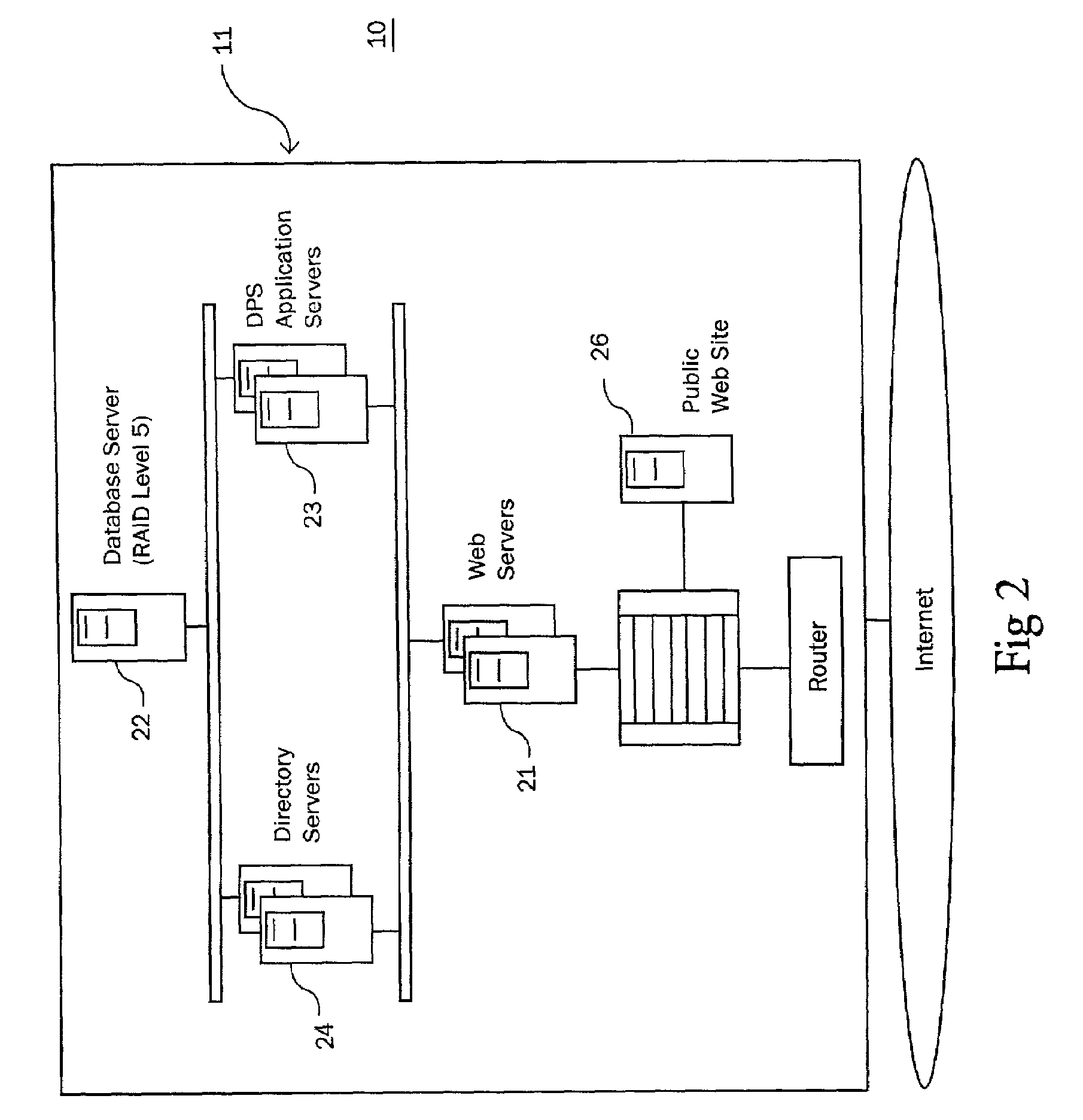
Owner:VIGILANZ CORP
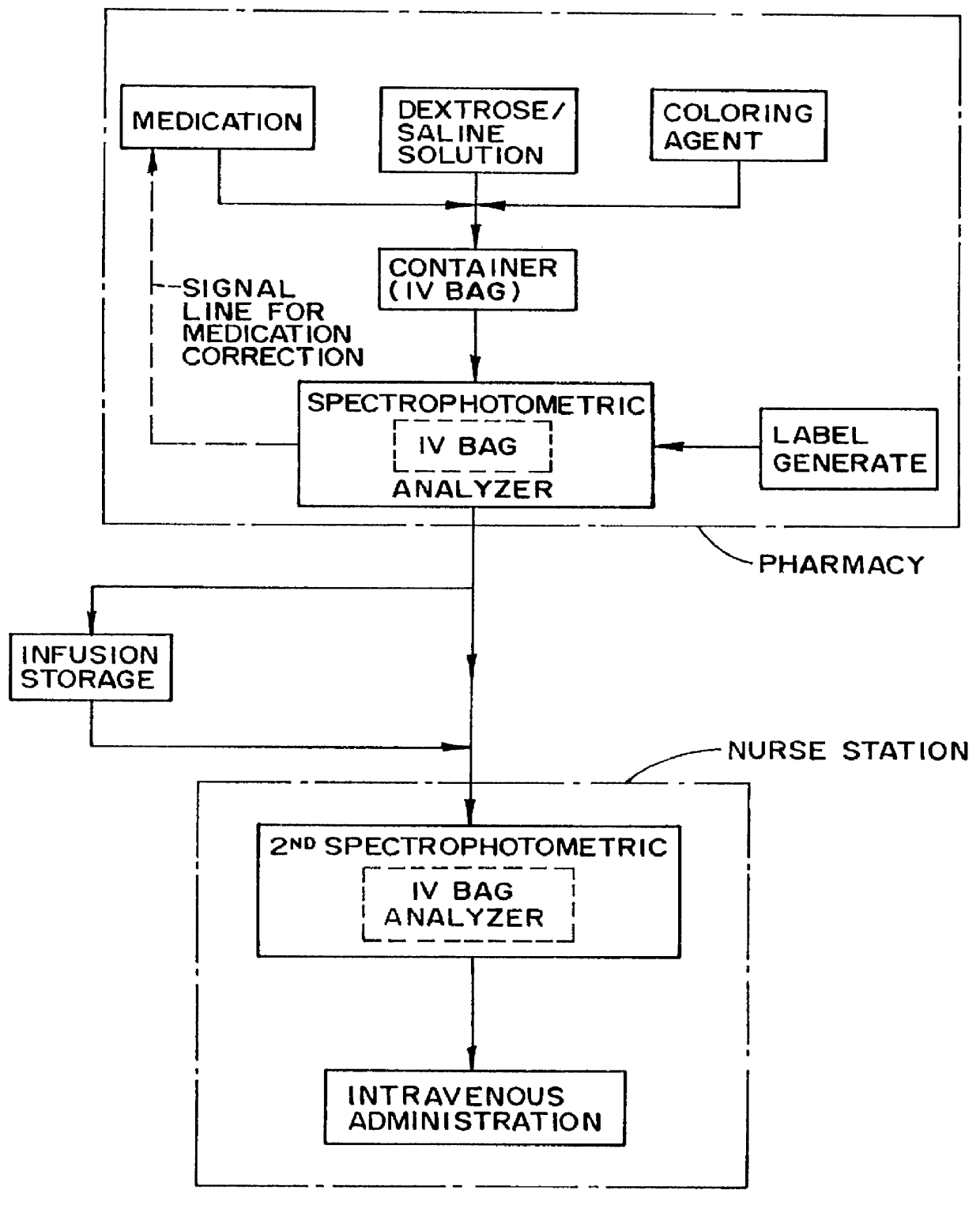
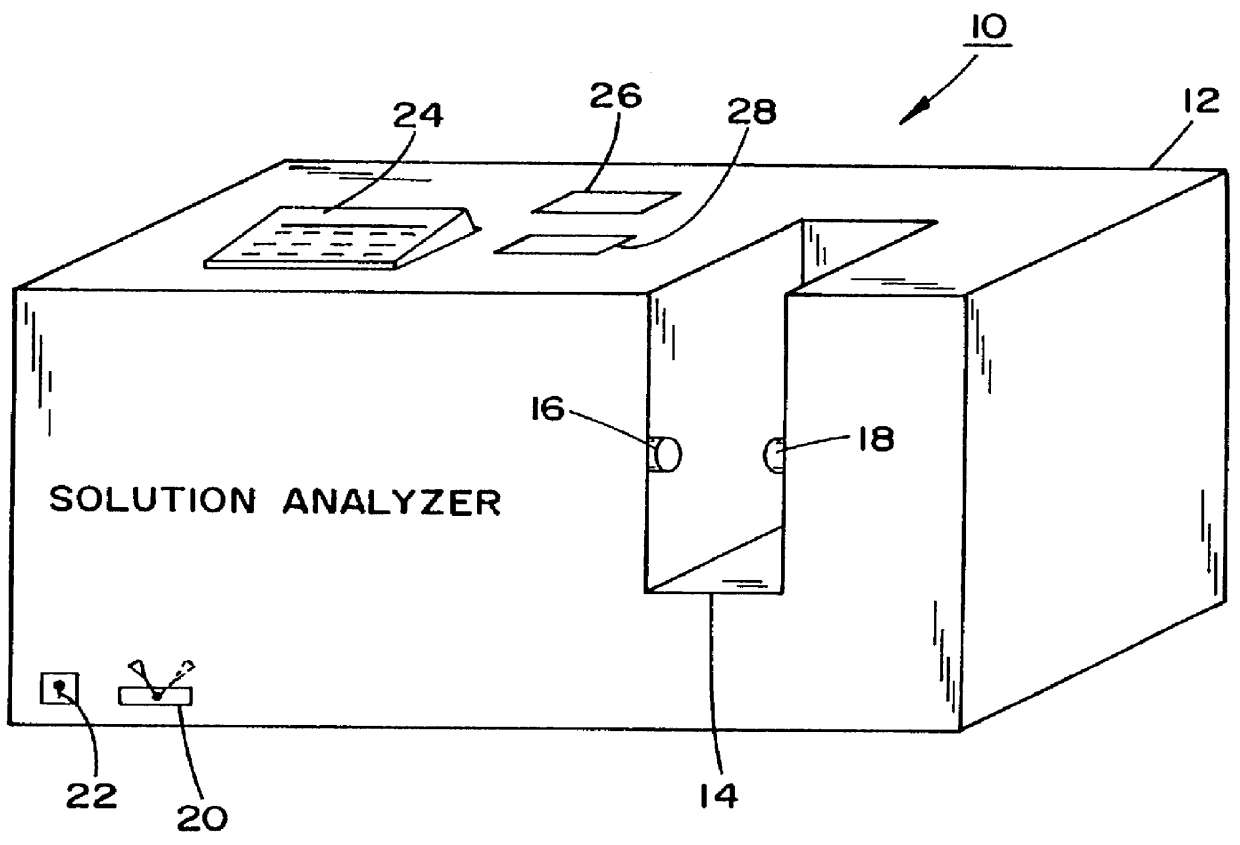
Method and apparatus for countering adverse drug events
InactiveUS6111639AReduction of adverse drug eventsReduce generationRadiation pyrometryDrug and medicationsPharmacyVein infusion
A method and apparatus for counteracting adverse drug events (ADE's) which are caused by the administration of intravenous medications of incorrect types, concentrations or dosages, and which may result in morbidity and even mortality to recipient patients. A container, preferably in the nature of a transparent plastic bag employed for intravenous administrations, contains the requisite infusion at a prescribed volume, adding a specified amount of prescribed medication possessing a predefined amount of a coloring material, such as a vegetable dye, to the volume of infusion material or liquid in order to form a prescribed concentration of medication, with the coloring material defining a specific type of medication. Analyzing of the concentration of medication is effected through the utilization of spectrophotometric equipment, and to resultingly obtain a real medication concentration value. Thereafter, a comparison is carried out between the real or actual and the prescribed medication concentration values, and an indicator, such as a label or the like, is generated for placement on the intravenous container which is indicative of the type and verified concentration of the medication in the infusion. This procedure, in effect, by being implemented at the pharmacy level, provides for medication and concentration verification prior to administering the medication intravenously to a patient, and by verifying the presence of the indicator on the intravenous (IV) container, or by using a spectrophotometer at a nurse station in order to again verify the medication and its concentration as a check or safeguard preceding administration thereof to the patient.
Owner:REDUTO LAWRENCE A
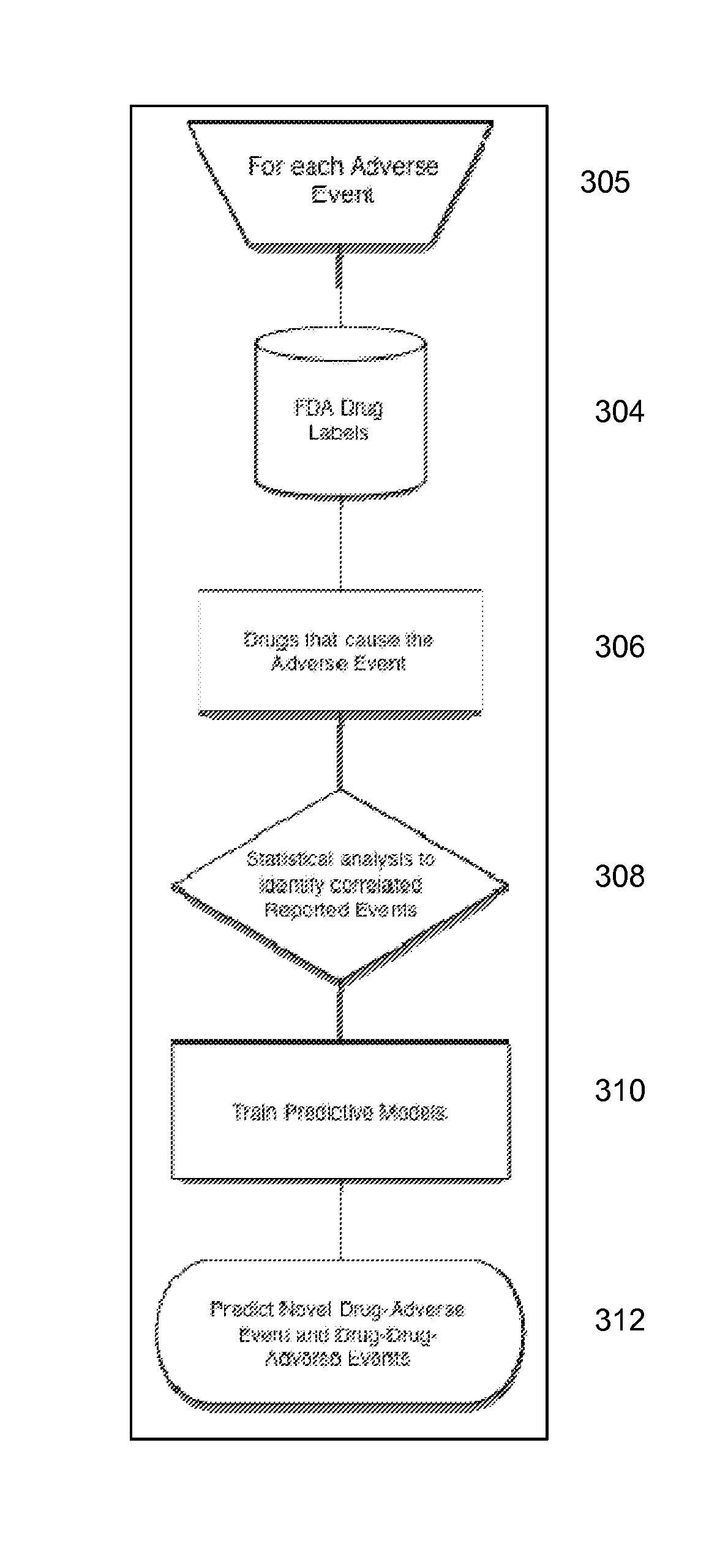
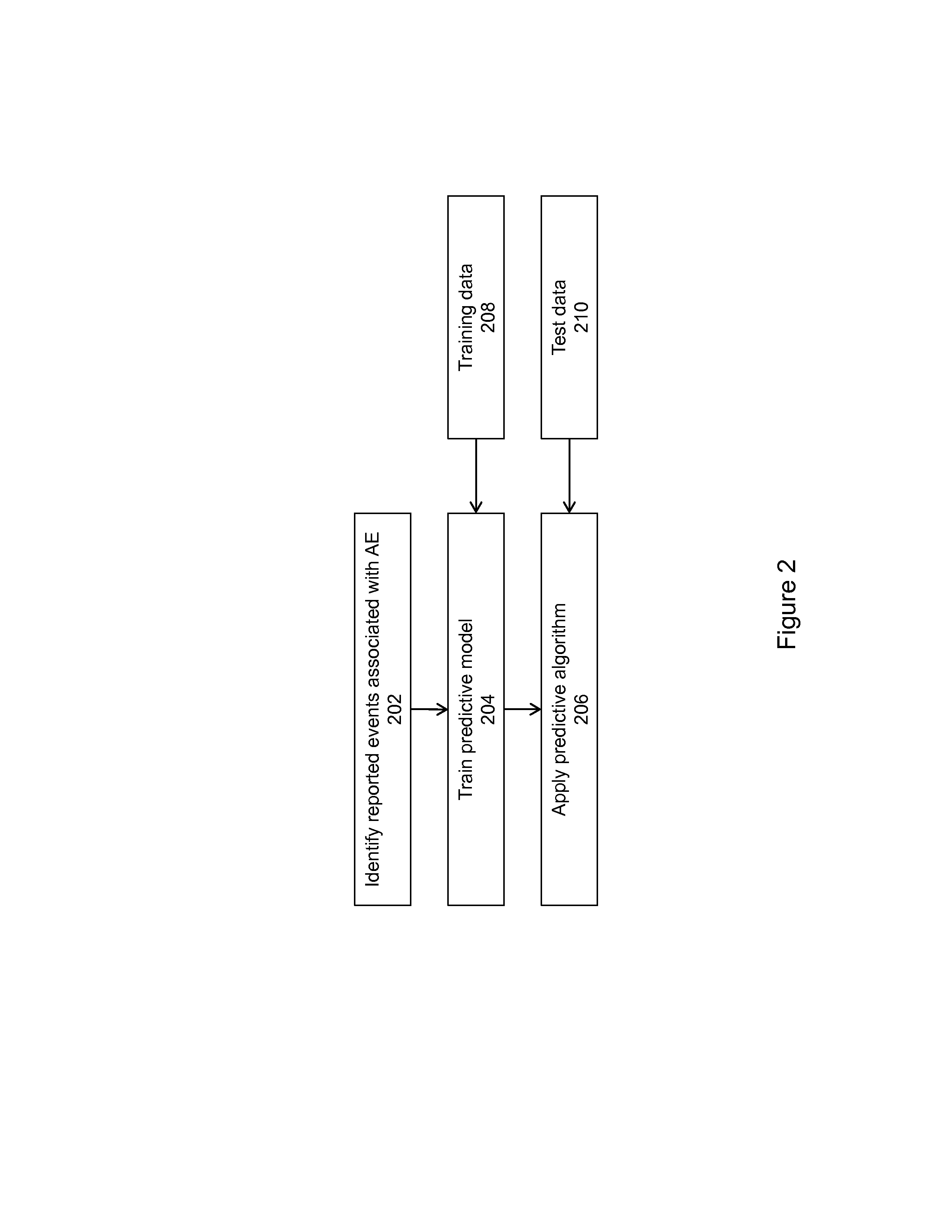
Signal Detection Algorithms to Identify Drug Effects and Drug Interactions
ActiveUS20130179375A1Improve performanceMedical simulationDrug and medicationsObservational databaseDrug interaction
An algorithm according to an embodiment of the present invention provides for latent signal detection of adverse events. Embodiments infer the presence of adverse drug events from large observational databases housed by the FDA, WHO, and other governmental organizations. The disclosed algorithms do not require the adverse event to be reported explicitly. Instead, the algorithms infer the presence of adverse events through more common secondary effects. In an embodiment, machine learning techniques are used for this purpose.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Method and system for identifying and anticipating adverse drug events
The present invention is a system and method for anticipating potential Adverse Drug Events (ADE) in a patient's medication regimen by integrating data typically located in laboratory and pharmacy information systems and filtering the data using predefined criteria. The present invention includes a system for anticipating a possible ADE through the use of a search engine that compares integrated data from laboratory and pharmacy information systems and compares it to predefined ADE rules defining normal ranges for a particular laboratory test. If an abnormal test value is received and a drug in the patient's medication regimen satisfies a drug included in an ADE rule then an alert procedure is triggered which allows for a period of time wherein the patient's lab and pharmacy data is monitored in order to determine if a proper corrective action is undertaken, and if no corrective action or an improper corrective action is taken within that period of time, the healthcare provider is warned of a potential ADE.
Owner:KLASS DAVID B +2
Method of mitigating adverse drug events using omega-3-fatty acids as a parenteral therapeutic drug vehicle
A method of parenterally administering a composition, the method including parenterally administering to a person a composition including at least one omega-3 fatty acid and at least one drug, wherein the at least one omega-3 fatty acid source and the at least one drug are administered simultaneously.
Owner:STABLE SOLUTIONS
Dual smart card e-prescription system and method
InactiveUS20150213204A1Data processing applicationsDrug and medicationsParticipating OrganizationSmart card
A dual smart card system and method including a central exchange server which collects patient healthcare data from a plurality of healthcare providers and stores the data in its central data bases. Each healthcare provider and each patient has a smart card and both smart cards must be used when a provider prescribes a medication for the patient and when the pharmacy fills the prescription for the patient. The patients' medical information is stored on the patient's smart card and on the central database. The system makes the stored medical information immediately available to participating organizations and tracks the entire life cycle of the prescription and generates alerts for adverse drug events, fraud, and duplication.
Owner:DUAL SMART CARD LLC
Method and system for identifying and anticipating adverse drug events
The present invention is a system and method for anticipating potential Adverse Drug Events (ADE) in a patient's medication regimen by integrating data typically located in laboratory and pharmacy information systems and filtering the data using predefined criteria. The present invention includes a system for anticipating a possible ADE through the use of a search engine that compares integrated data from laboratory and pharmacy information systems and compares it to predefined ADE rules defining normal ranges for a particular laboratory test. If an abnormal test value is received and a drug in the patient's medication regimen satisfies a drug included in an ADE rule then an alert procedure is triggered which allows for a period of time wherein the patient's lab and pharmacy data is monitored in order to determine if a proper corrective action is undertaken, and if no corrective action or an improper corrective action is taken within that period of time, the healthcare provider is warned of a potential ADE.
Owner:RING DENNIS J +2
Compositions for reducing risk of adverse events caused by drug-drug interactions
InactiveUS20120232066A1Reduce releaseReduce actionBiocideNervous disorderDrug interactionDrug-drug interaction
The present disclosure provides a composition comprising a GABAA agonist and a GI enzyme inhibitor. The present disclosure also provides a composition comprising (a) a GI enzyme inhibitor and (b) a first drug that interacts with a second drug to produce an adverse effect when the second drug is co-ingested as a GI enzyme-cleavable prodrug with the first drug. Such an interaction can be additive or synergistic.
Owner:SIGNATURE THERAPEUTICS
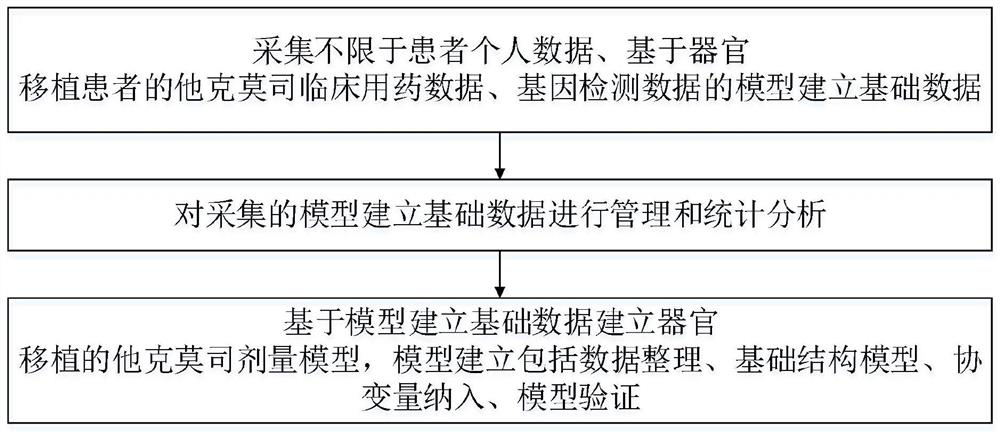
Accurate prediction method for tacrolimus dosage of organ transplantation patient
ActiveCN112786145AEliminate side effectsReduce riskMedical data miningDrug and medicationsImmunosuppressive drugDosage adjustment
The invention discloses an accurate prediction method for a tacrolimus dosage of an organ transplantation patient. The method comprises the following steps: collecting model establishment data including but not limited to patient personal data, tacrolimus clinical medication data based on the organ transplantation patient and gene detection data; carrying out management and statistical analysis on the collected model establishment data; and establishing a tacrolimus dosage model for organ transplantation based on the model establishment data, wherein the model establishment comprises data arrangement, basic structure model, covariable incorporation and model verification. The tacrolimus accurate dosage prediction mathematical model helps clinically and accurately predict the initial medication dosage and dosage adjustment scheme of an individual patient, reduces toxic and side effects caused by too large tacrolimus dosage or the risk of rejection caused by too low tacrolimus dosage of the patient, reduces the occurrence rate of acute rejection reaction and reduces adverse drug events. The prediction method has important significance on accurate application of immunosuppressive drugs for organ transplantation patients; meanwhile, has good economic benefits and social benefits.
Owner:XIEHE HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI & TECH UNIV
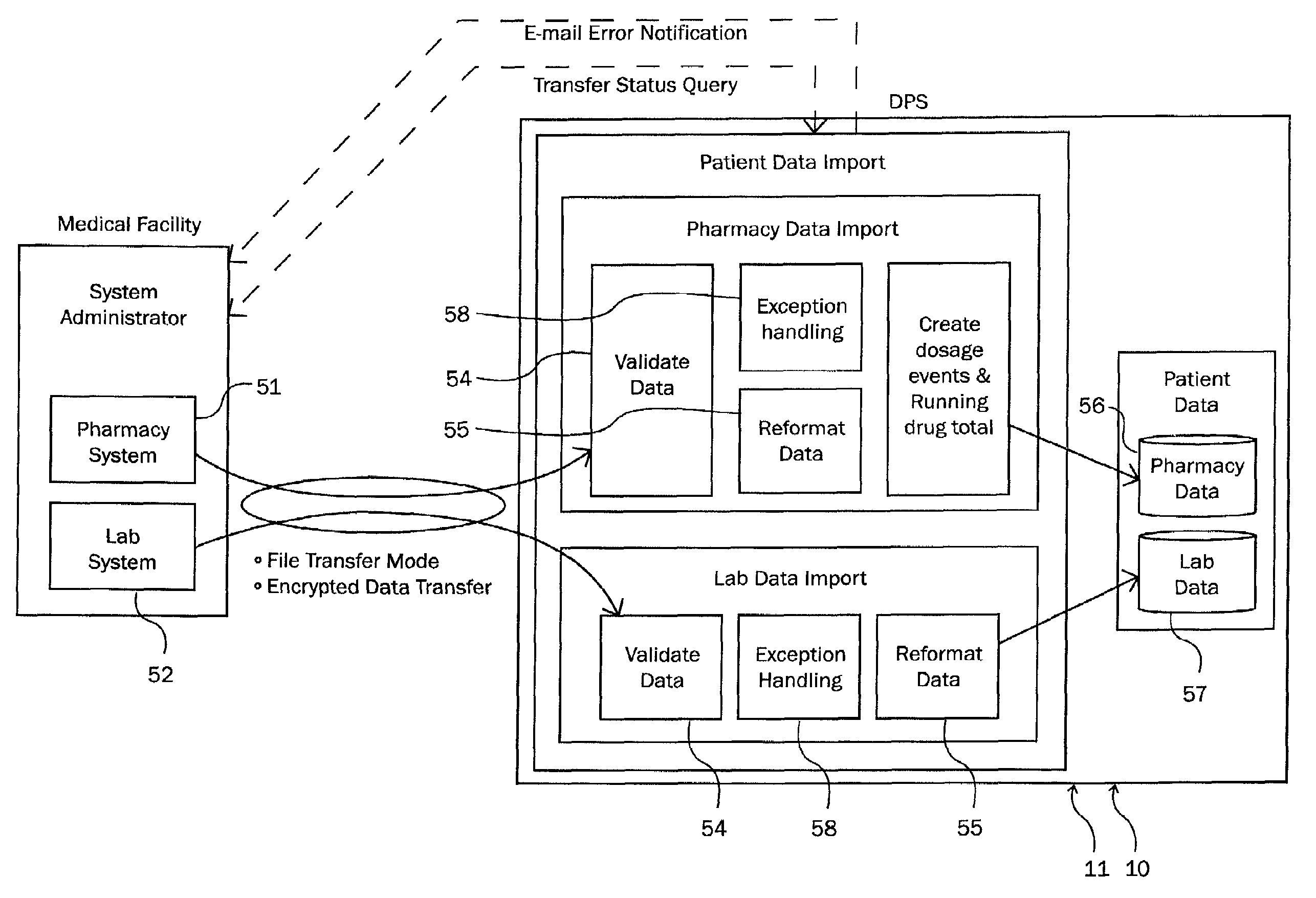
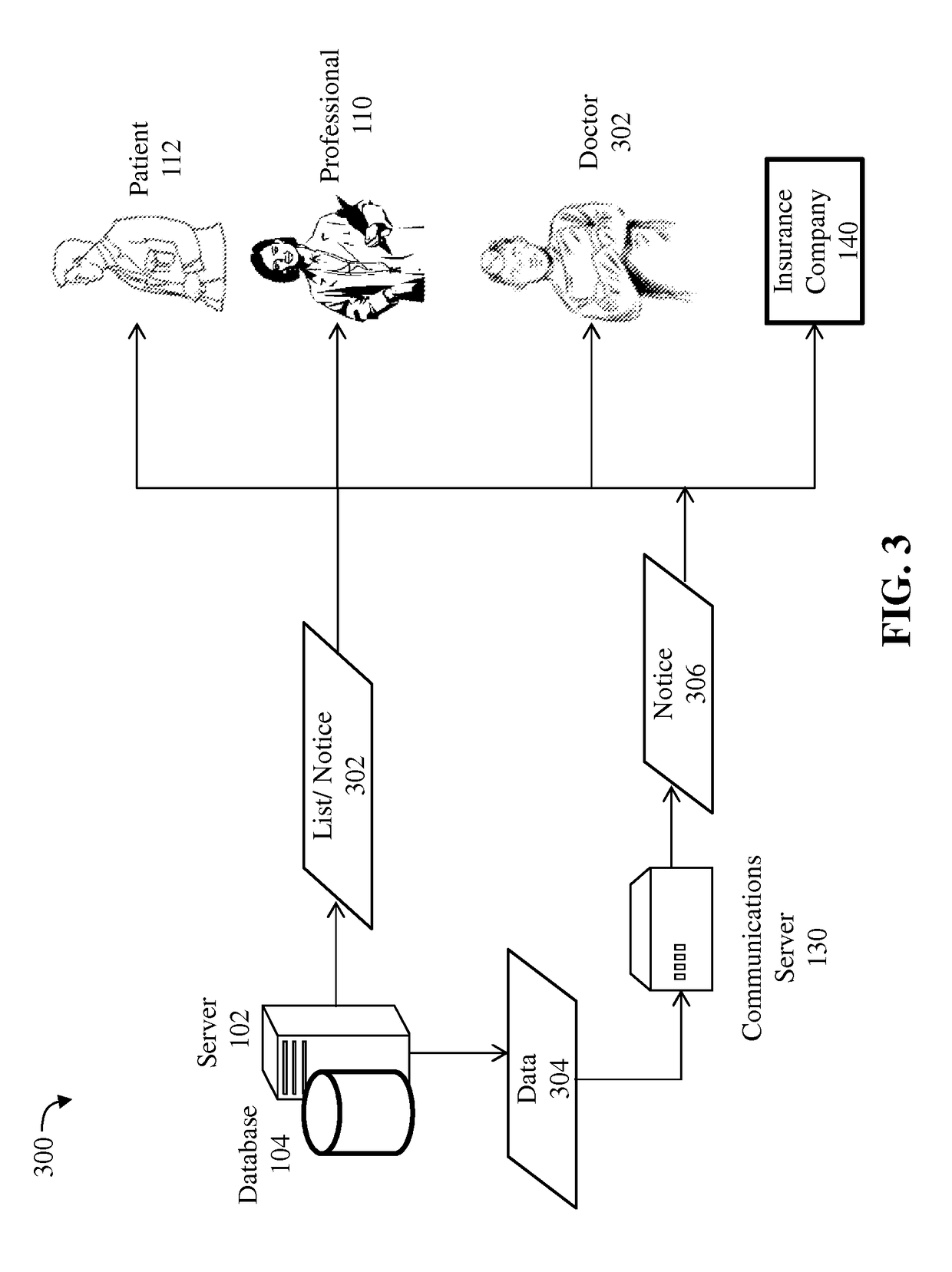
Detection of adverse reactions to medication using a communications network
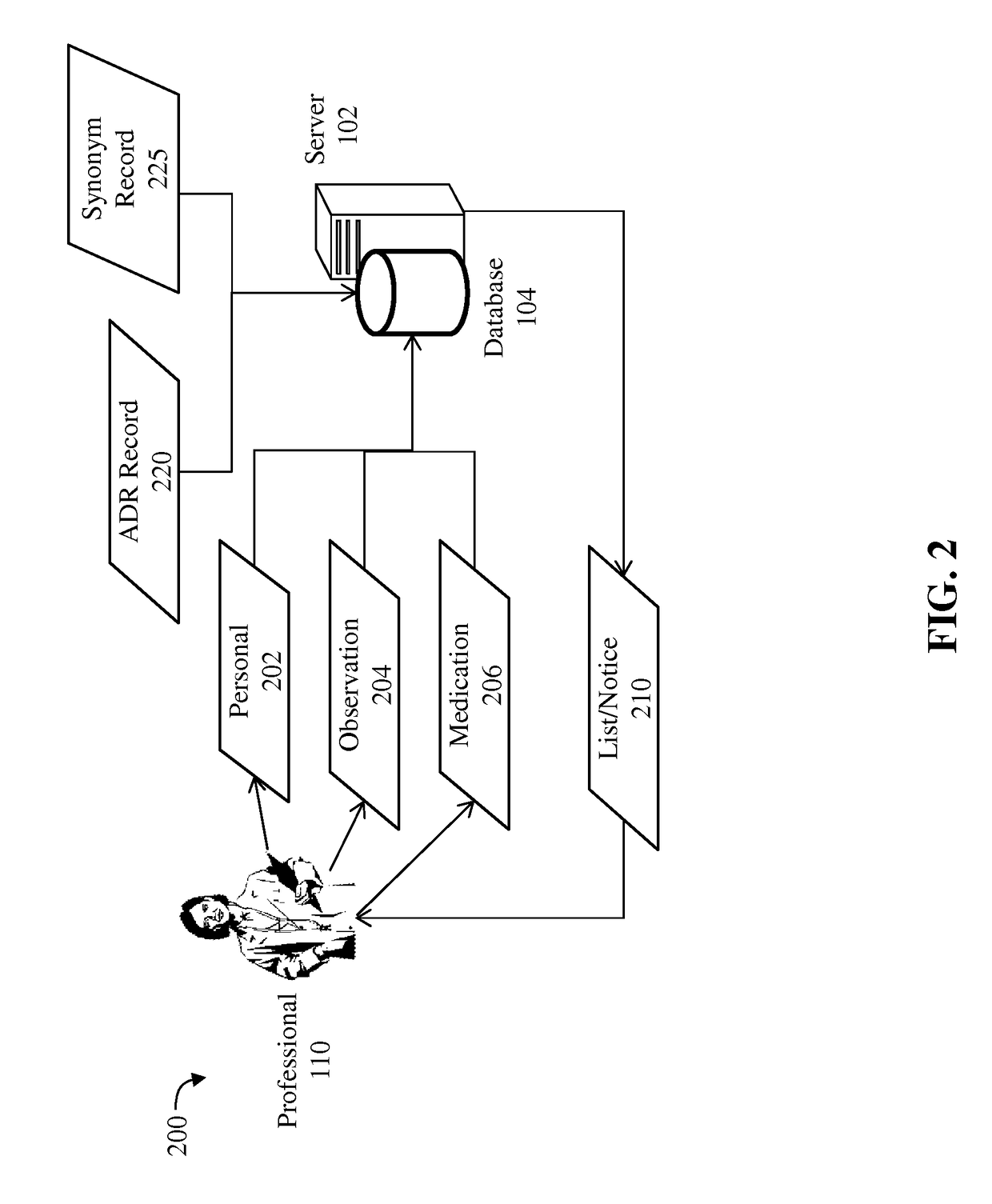
A method for detecting an adverse drug event in a patient. The method includes reading an adverse drug reaction (ADR) description of the patient, reading identifiers for medications currently taken by the patient, and accessing ADR records corresponding to the identifiers. Each record comprises an identifier for a medication, an ADR description for the medication and a risk profile for the medication. Next, the method compares the ADR description of the patient with ADR descriptions in the ADR records, identifies an ADR record with a matching ADR description and calculates a level of risk that a medication of the ADR record is causing the ADR description of the patient. Finally, the method transmits an identifier and a risk profile for each medication of the ADR record, and a notice indicating that each medication of the ADR record may be causing the ADR description of the patient.
Owner:LUCCHINO RONALD
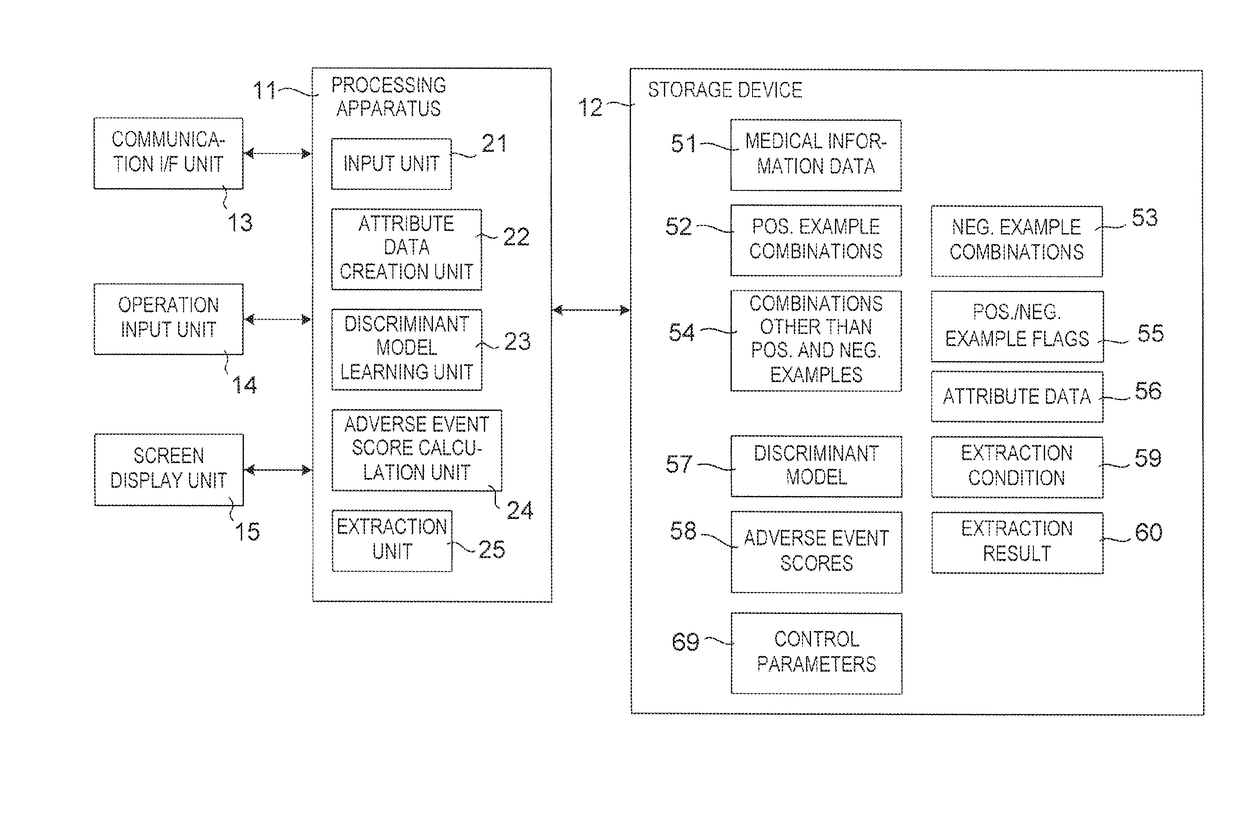
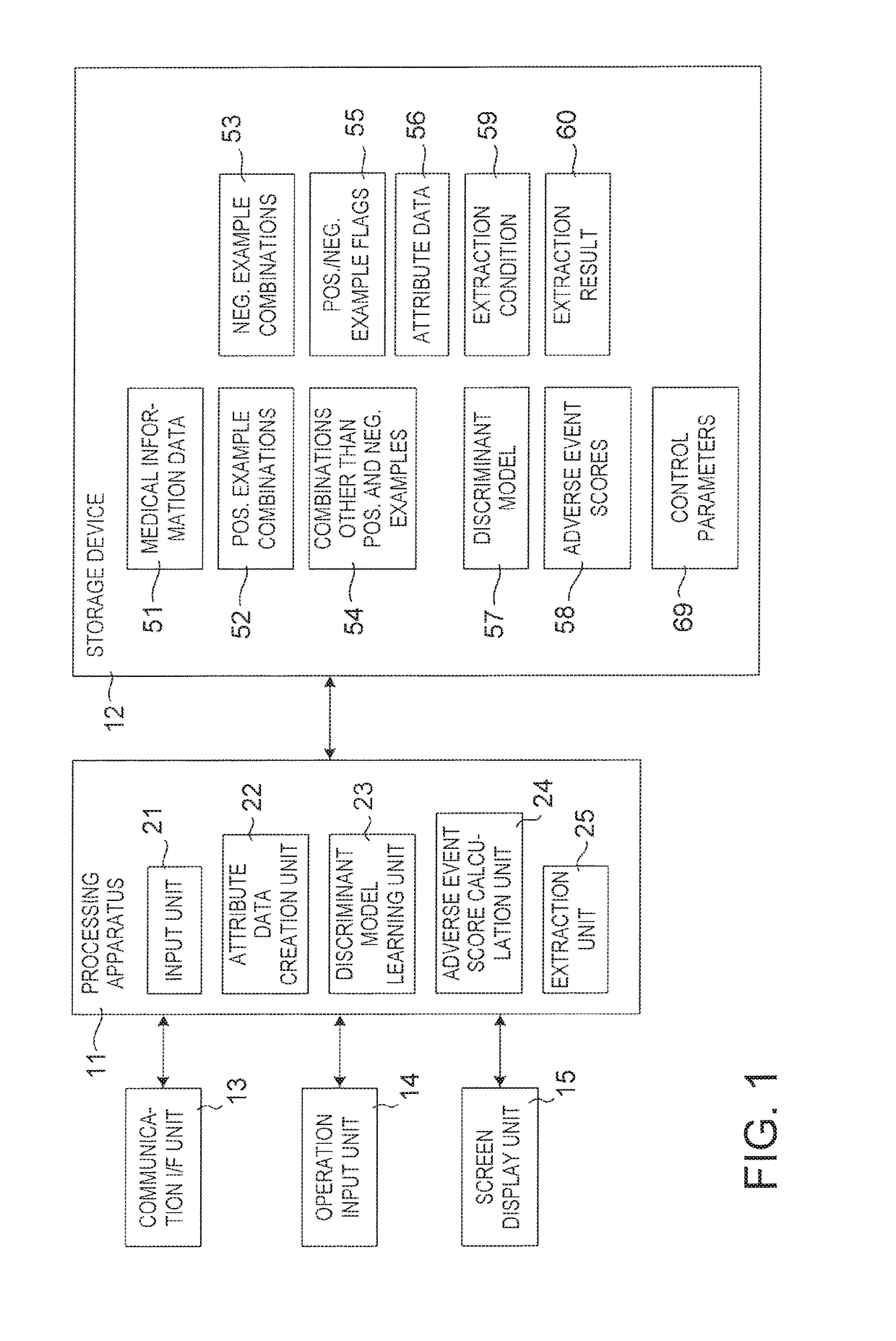
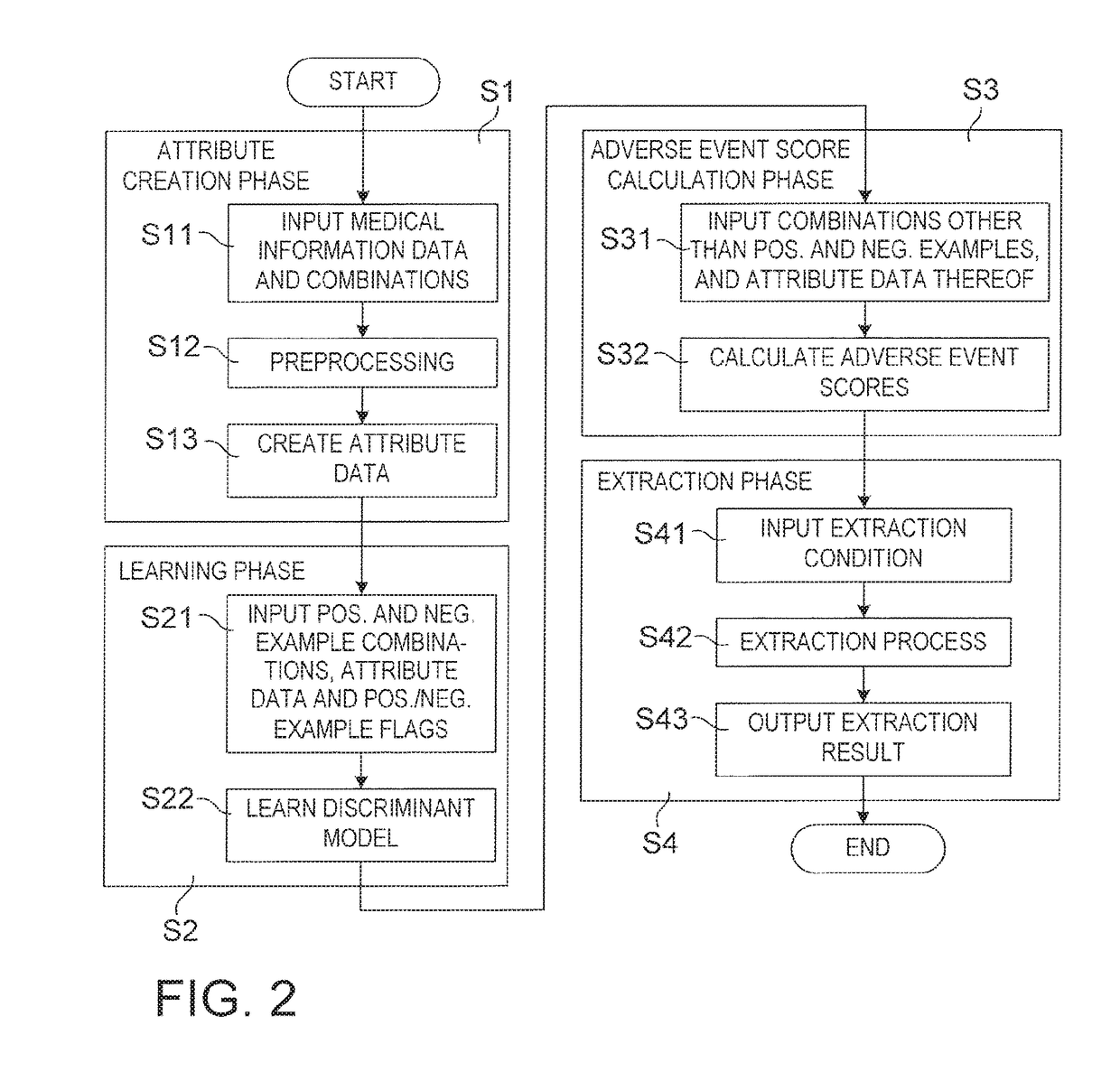
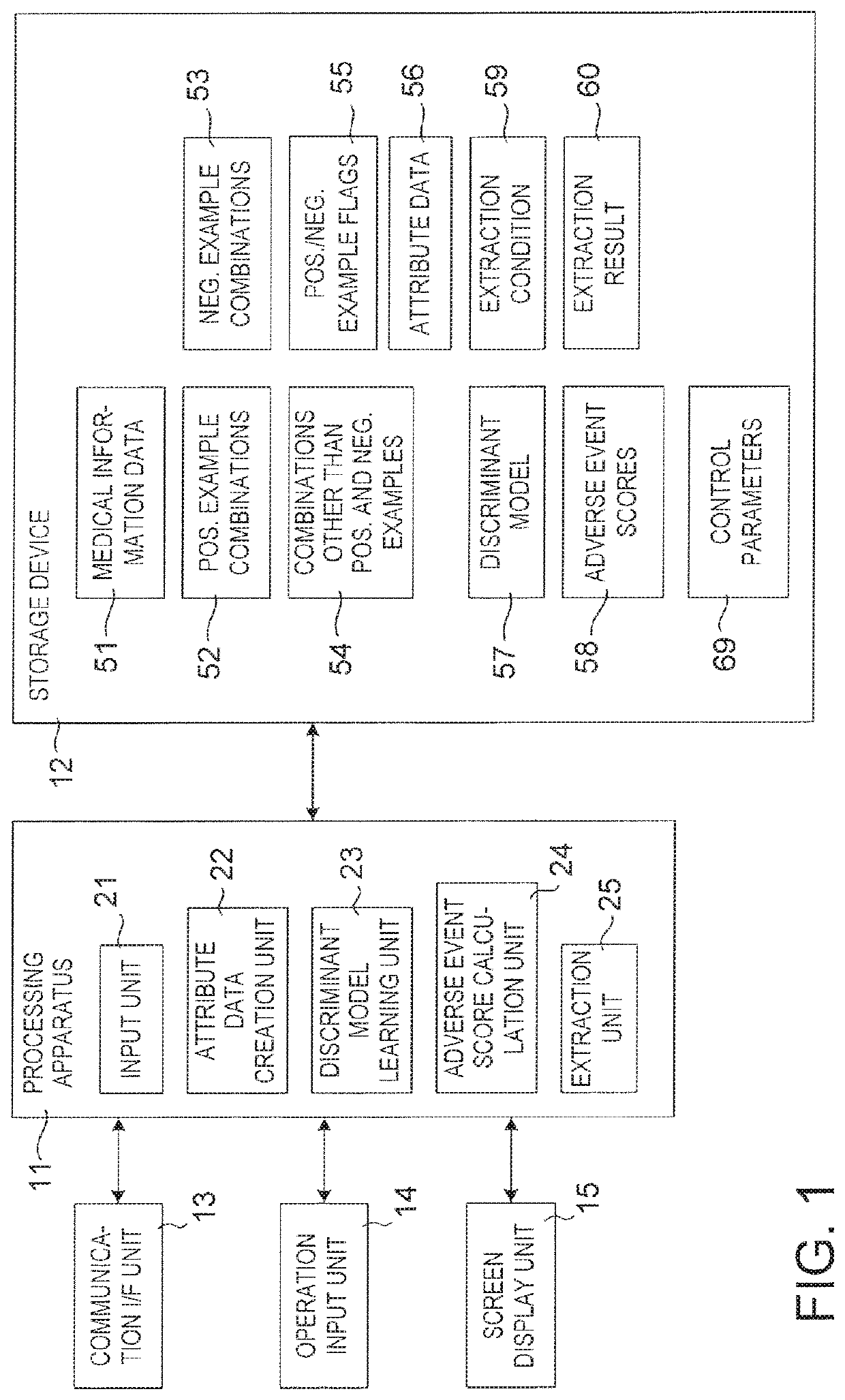
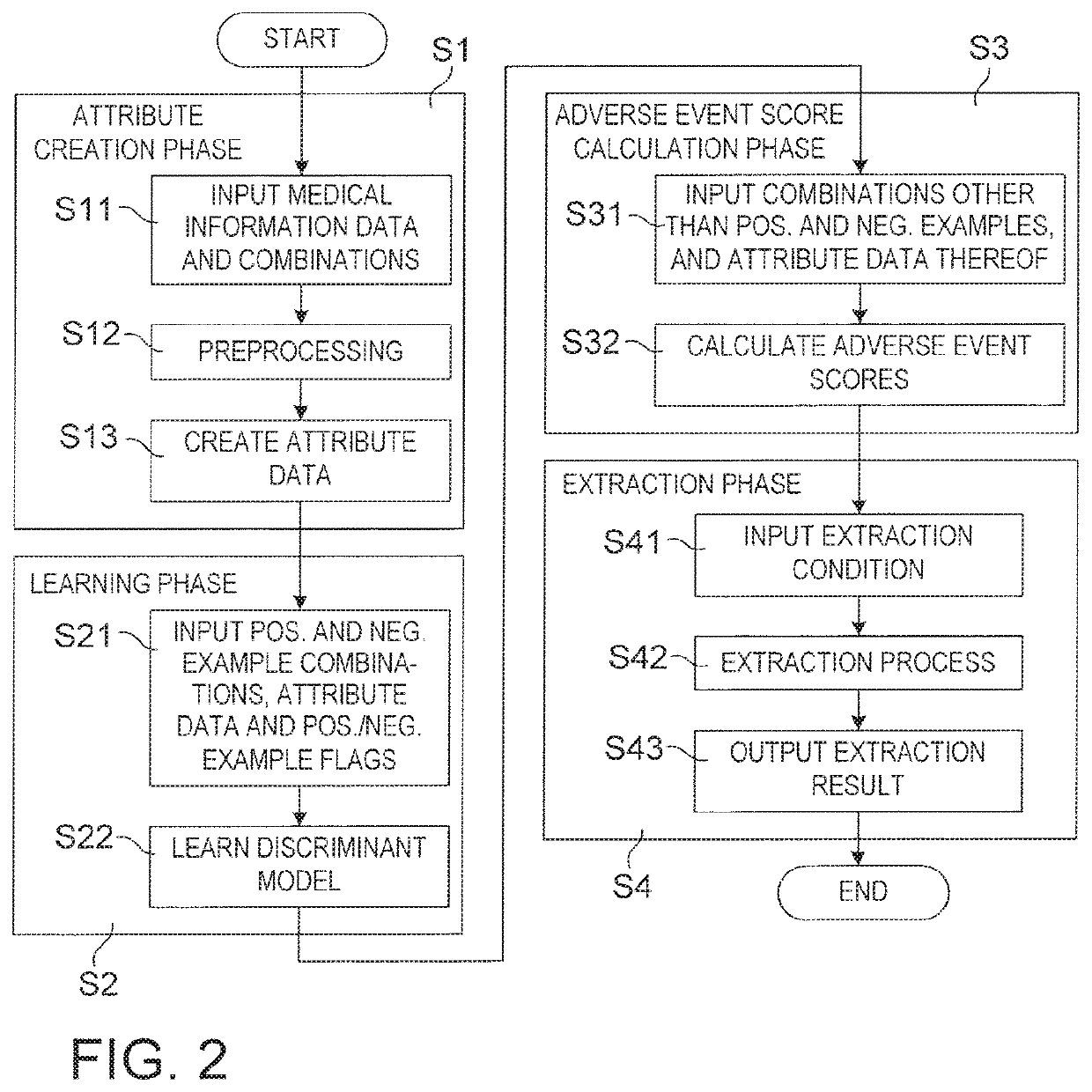
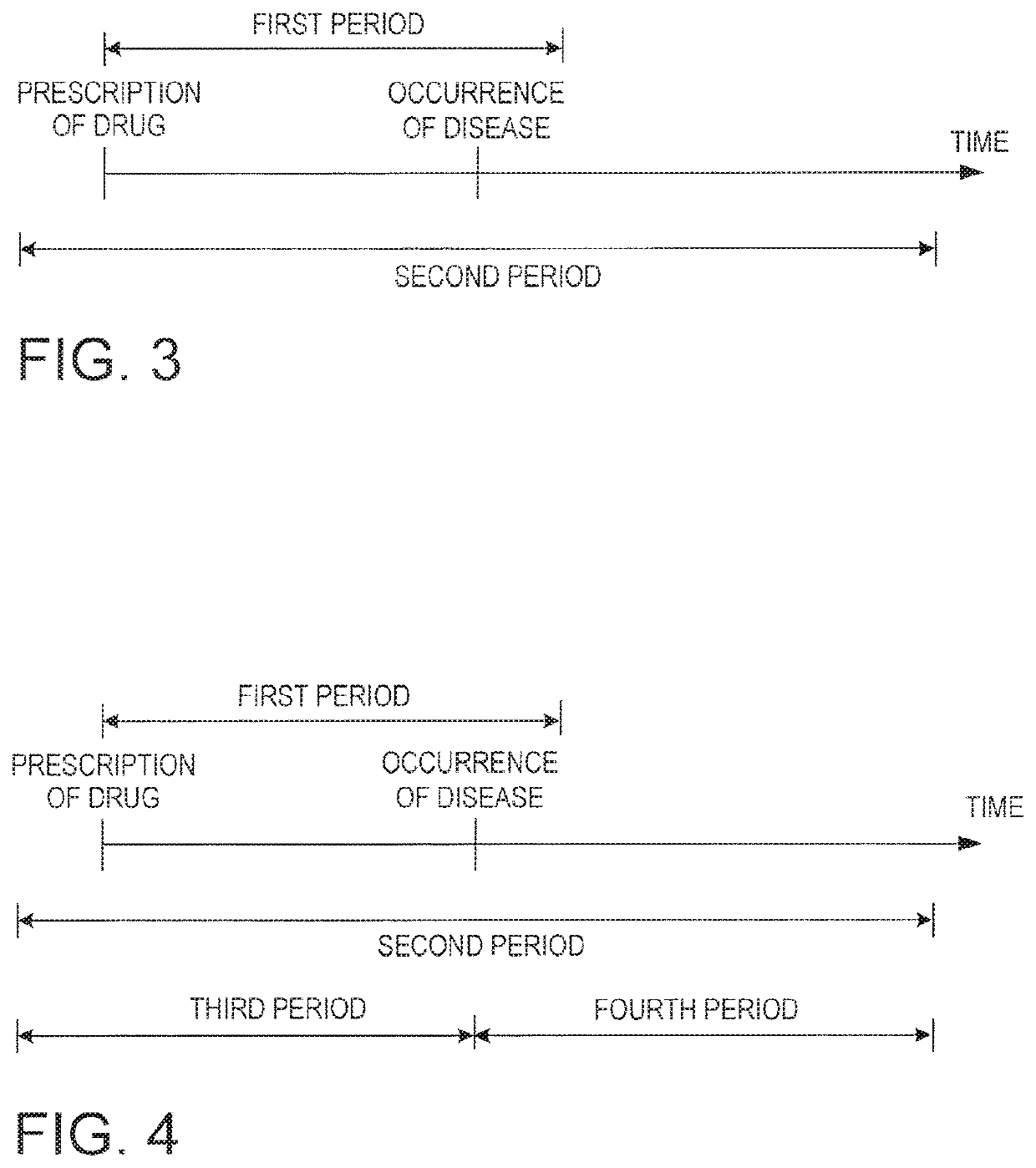
Drug adverse event extraction method and apparatus
ActiveUS20170083670A1Accurate extractionMedical automated diagnosisDrug referencesDiseaseDiscriminant model
A method of extracting a combination of a drug and an adverse event related to the drug includes: for each of positive example combinations, negative example combinations and combinations that are neither positive examples nor negative examples, which are combinations of drug and disease, extracting medical events from medical information data about a patient and generating attribute data based on time-series information about the medical events; and learning a discriminant model based on attribute data of the positive and negative examples; and inputting attribute data corresponding to the combinations that are neither positive examples nor negative examples to the discriminant model to determine scores.
Owner:NEC CORP
Signal detection algorithms to identify drug effects and drug interactions
ActiveUS9305267B2Improve performanceMedical simulationDrug and medicationsObservational databaseDrug interaction
An algorithm according to an embodiment of the present invention provides for latent signal detection of adverse events. Embodiments infer the presence of adverse drug events from large observational databases housed by the FDA, WHO, and other governmental organizations. The disclosed algorithms do not require the adverse event to be reported explicitly. Instead, the algorithms infer the presence of adverse events through more common secondary effects. In an embodiment, machine learning techniques are used for this purpose.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
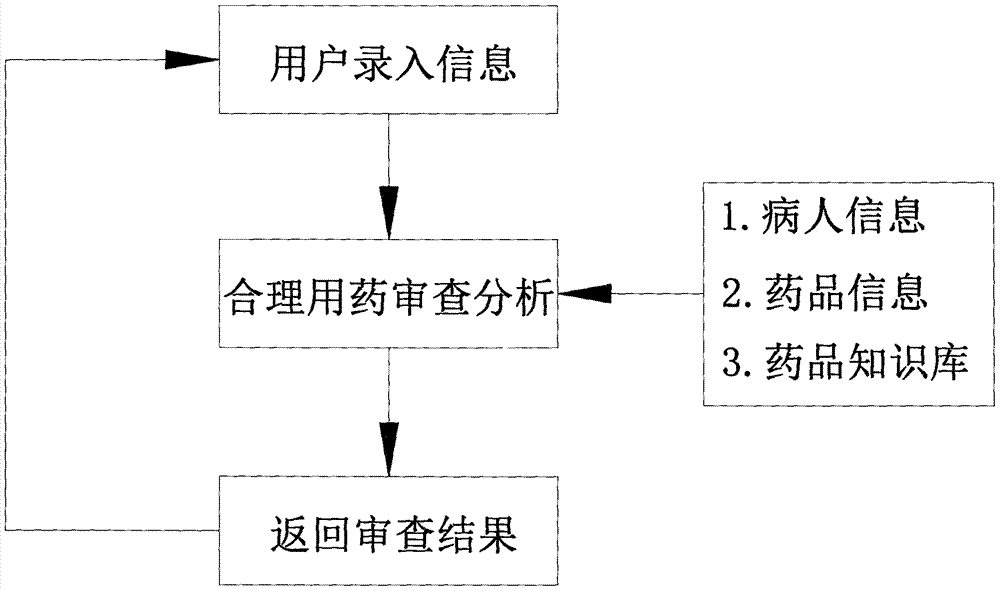
Method for reading prescription drug properties by using computer
PendingCN107391897ATimely and effective graspTimely and effective useSpecial data processing applicationsMedication PrescriberPharmacist
The invention discloses a method for reading prescription drug properties by using a computer. The method comprises a knowledge database, information input by a user and reasonable drug use review analysis; after the information input by the user and the reasonable drug use review analysis, an analysis result is fed back to a user information input interface; the reasonable drug use review analysis is comparative analysis with the knowledge database; and the knowledge database comprises patient information, drug information and a drug knowledge library. According to the method, automatic review of prescriptions and medical advices is realized; potential unreasonable drug use problems are discovered in time; clinical professionals such as doctors, pharmacists and the like are assisted to master and use medical knowledge effectively in time in a drug use process; the occurrence of adverse drug events is prevented; the technical progress of clinical reasonable drug use work is promoted; and the occurrence of medical events is reduced.
Owner:广州市保进网络科技有限公司
Application of traditional Chinese medicine composition in preparation of medicine for treating novel coronavirus pneumonia
InactiveCN112999323ATCM syndrome score improvementSignificant symptom relief rateAntinoxious agentsAntiviralsConvalescenceWolfiporia extensa
The invention relates to an application of a traditional Chinese medicine composition in preparation of a medicine for treating novel coronavirus pneumonia, in particular to an application in preparation of a medicine for treating a lung-spleen qi deficiency syndrome in a clinical treatment recovery phase of the novel coronavirus pneumonia. The traditional Chinese medicine composition is prepared by compatibility of thirteen traditional Chinese medicinal materials including prepared rehmannia root, radix aconiti lateralis praeparata (black prepared aconite root), moutan bark, radix achyranthis bidentatae, salted fructus psoraleae, fructus amomi, semen plantaginis, poria cocos, salted fructus alpiniae oxyphyllae, cinnamon, Chinese yam, rhizoma alismatis, fructus rosae laevigatae and the like according to the principle of monarch, minister, assistant and guide. The medicines are used for warming kidney, absorbing qi, tonifying spleen and reducing phlegm together. Clinical test results show that the traditional Chinese medicine composition can significantly improve the traditional Chinese medicine syndrome integral of a patient in the convalescent stage of the new crown pneumonia, and has significant symptom remission rate and relatively fast symptom remission time for new crown pneumonia symptoms such as weakness, cough and the like. In addition, the composition is free of adverse drug events and good in safety.
Owner:GUANGZHOU BAIYUNSHAN JINGXIUTANG PHARM CO LTD
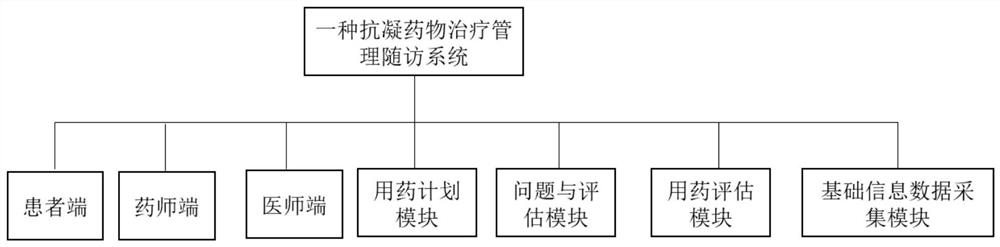
Anticoagulant drug treatment management follow-up visit system
PendingCN113593724AEasy to viewAvoid callingMedical communicationDrug and medicationsTreatment managementData acquisition
The invention relates to the technical field of medical data processing systems, in particular to an anticoagulant drug treatment management follow-up visit system which comprises a patient basic information data acquisition module, a medication evaluation module, a problem and evaluation module, a medication planning module, a physician end, a pharmacist end and a patient end. By adopting the system provided by the invention, the working efficiency of medical staff is improved, analysis and suggestion are carried out according to the medication condition of the patient, the safety of the patient when the patient uses the anticoagulant drug is enhanced, the medication condition of the patient is standardized, meanwhile, the medical staff can conveniently know the medication condition of the patient in time, and adverse drug events are reduced.
Owner:SICHUAN ACADEMY OF MEDICAL SCI SICHUAN PROVINCIAL PEOPLES HOSPITAL
Method of mitigating adverse drug events using omega-3 fatty acids as a parenteral therapeutic drug vehicle
Owner:STABLE SOLUTIONS
Application of traditional Chinese medicine composition in preparation of anti-novel coronavirus drug
InactiveCN112316032AStable conditionHigh rate of symptom reliefAnthropod material medical ingredientsAntiviralsInflammatory factorsClinical tests
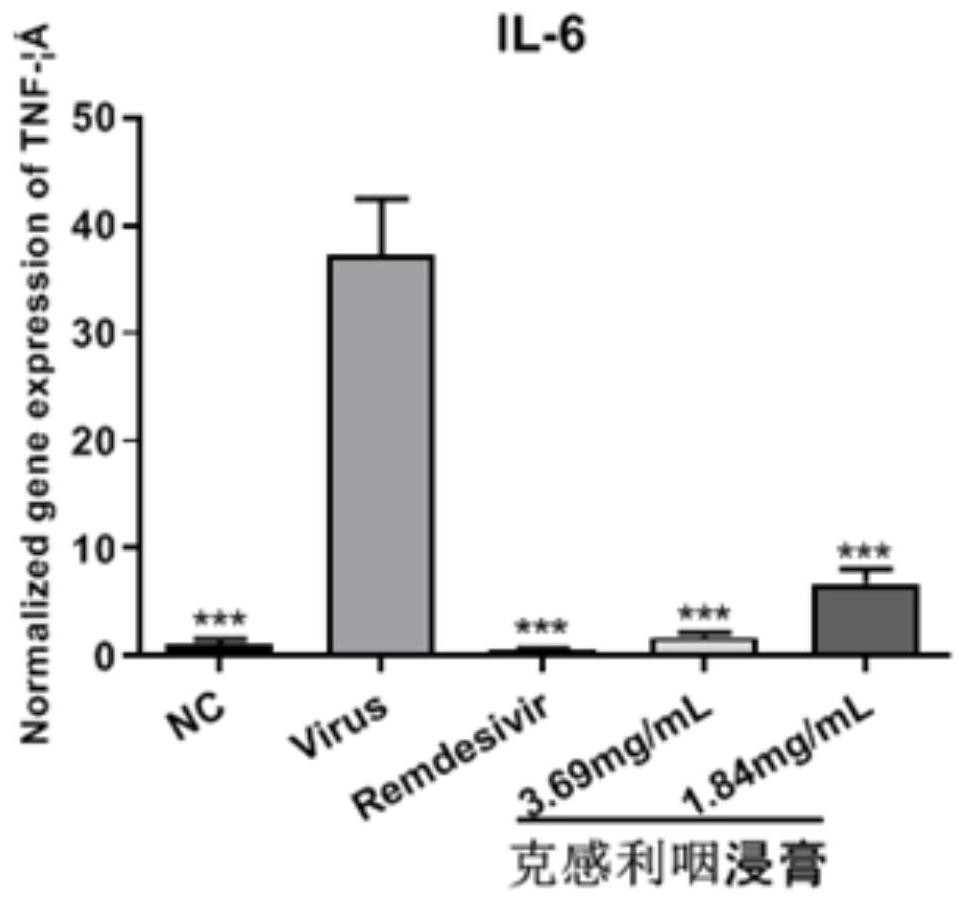
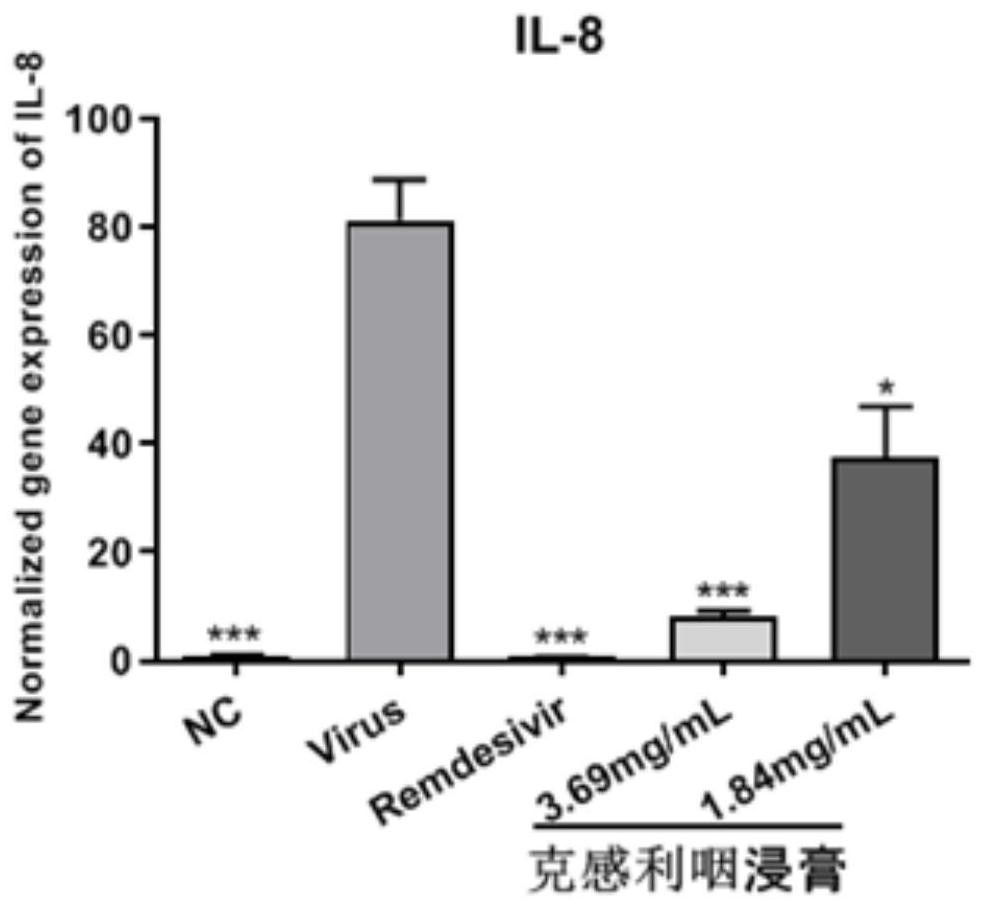
The invention provides application of a traditional Chinese medicine composition in preparation of an anti-novel coronavirus drug. The traditional Chinese medicine composition is a Kegan sore-throat relieving composition, and in-vitro experimental results show that the anti-novel coronavirus effect means that the composition can inhibit Vero E6 cells infected with novel coronavirus from causing cytopathy and inhibit mRNA over-expression of inflammatory factors TNF-a, IL6, IL-8 and MCP-1 induced by SARS-CoV-2. Clinical test results show that the Kegan sore-throat relieving composition has a higher symptom relieving rate and shorter symptom relieving time for novel coronary pneumonia symptoms such as fever, cough and pharyngalgia and the like, and can stabilize the condition of a patient ina novel coronary pneumonia recovery period; and no adverse drug event occurs, and the safety is good.
Owner:GUANGZHOU WANGLAOJI PHARM CO LTD
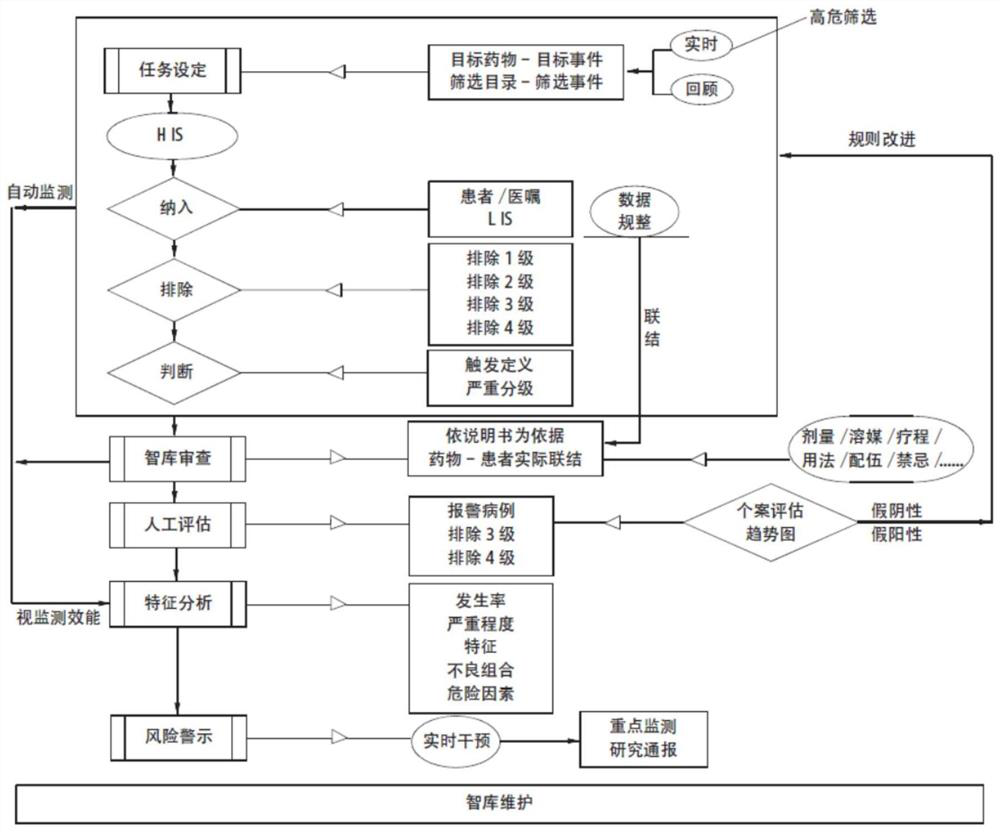
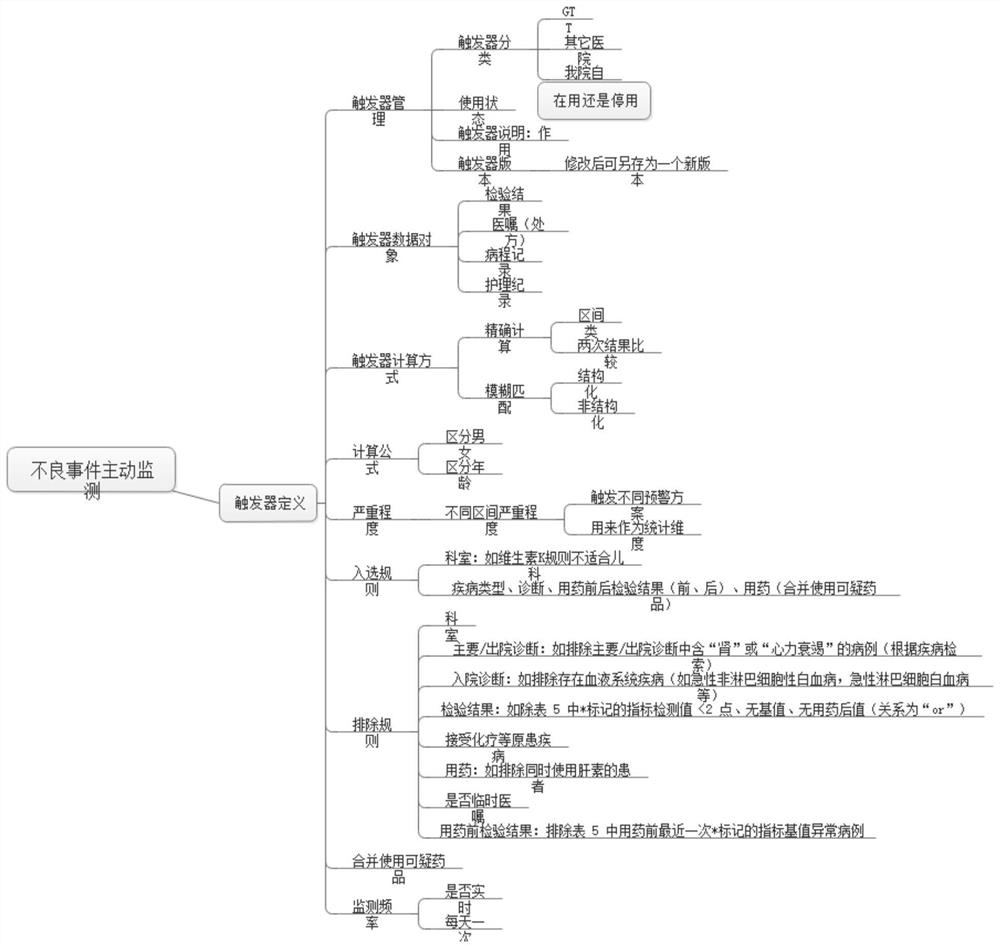
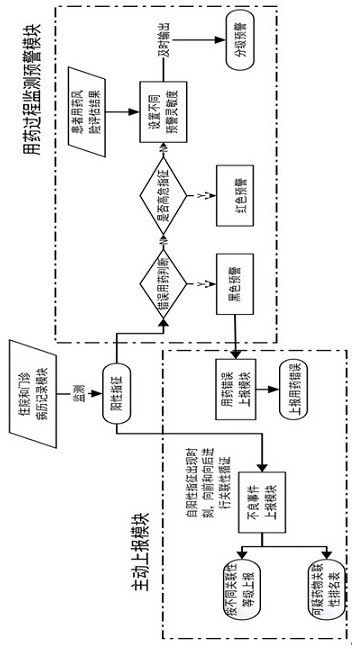
Active monitoring and evaluation warning system for adverse drug events of hospitalized patients
ActiveCN109285590ARapid assessmentHealth-index calculationDrug referencesMedication informationHospitalized patients
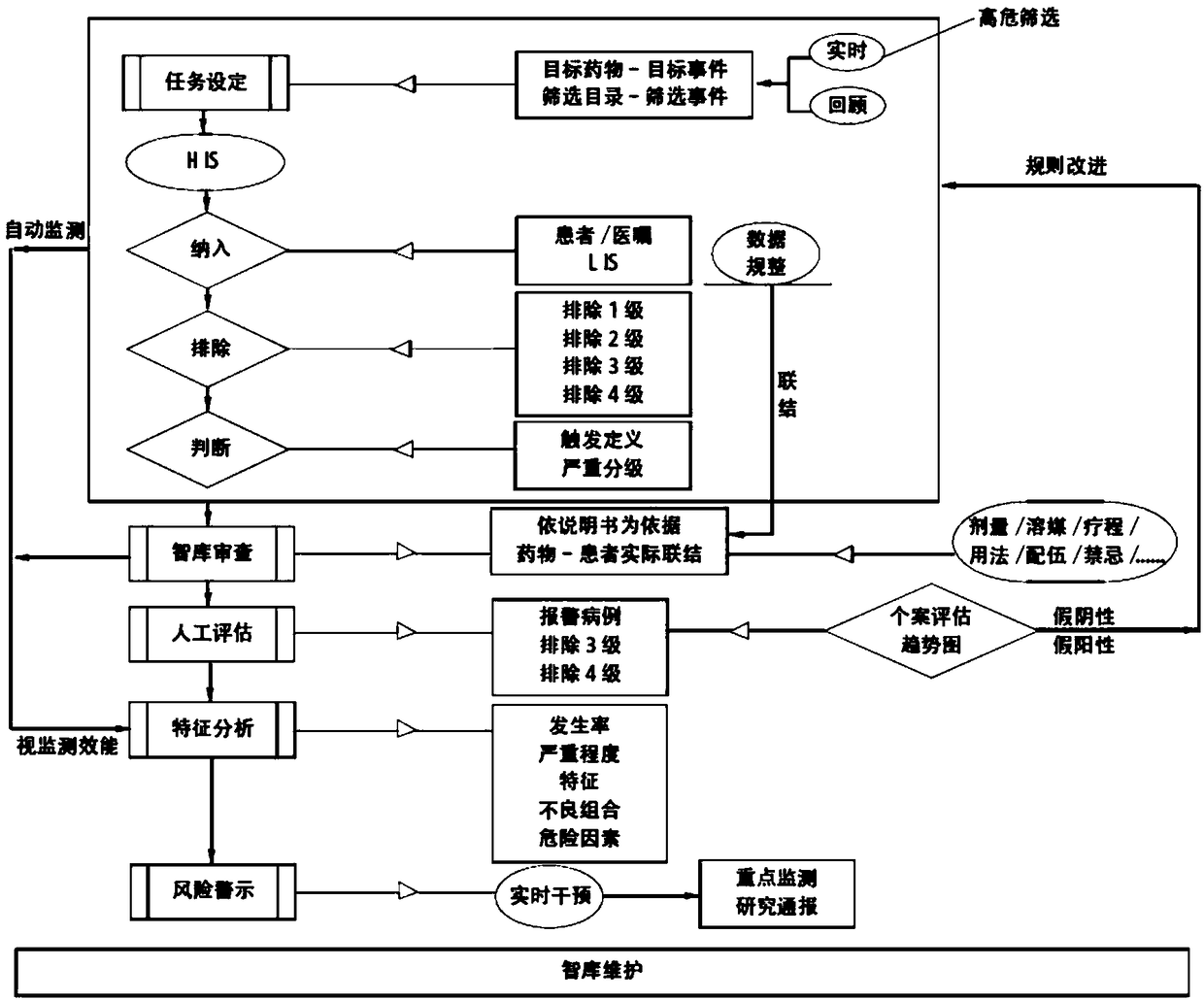
The invention provides an active monitoring and evaluation warning system for adverse drug events in hospitalized patients. Before the system is set up, a plurality of linked servers and a reporting system are set up. The reporting system transmits the patient's medication information and doctor's orders to the plurality of linked servers; a think tank is formed according to the set rules; after the completion of the think tank construction, the system includes a task setting module, an HIS module, an inclusion module, an exclusion module, a judgment module, a think tank examination module, amanual evaluation module, a feature analysis module, and a risk warning module; and the task setting module, the HIS module, the inclusion module, the exclusion module, the judgment module, the thinktank examination module, the manual evaluation module, the feature analysis module, and the risk warning module are sequentially connected.
Owner:广州火龙果信息科技有限公司
Method of mitigating adverse drug events using omega-3 fatty acids as a parenteral therapeutic drug vehicle
A method of parenterally administering a composition, the method including parenterally administering to a person a composition including at least one omega-3 fatty acid and at least one drug, wherein the at least one omega-3 fatty acid source and the at least one drug are administered simultaneously.
Owner:STABLE SOLUTIONS
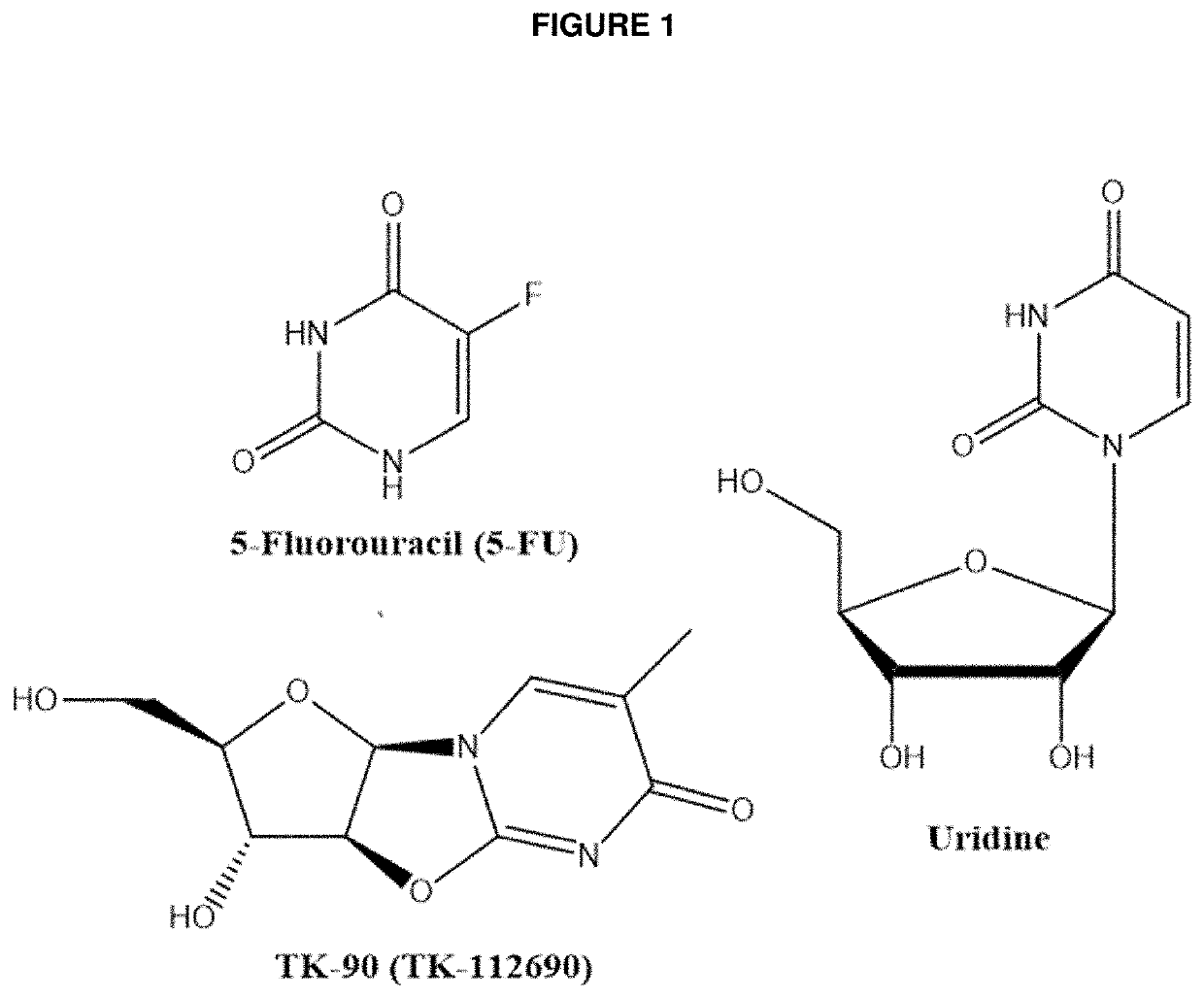
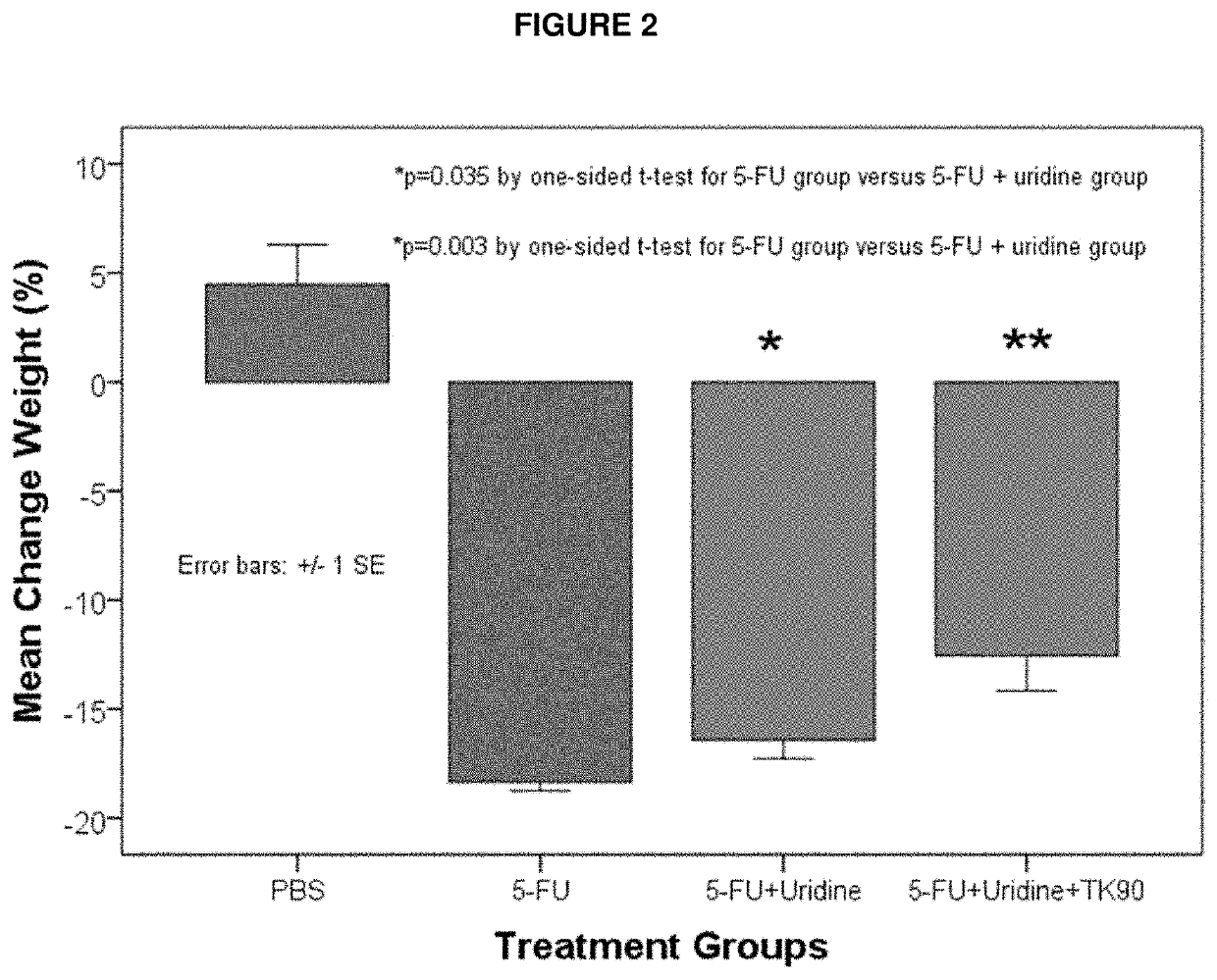
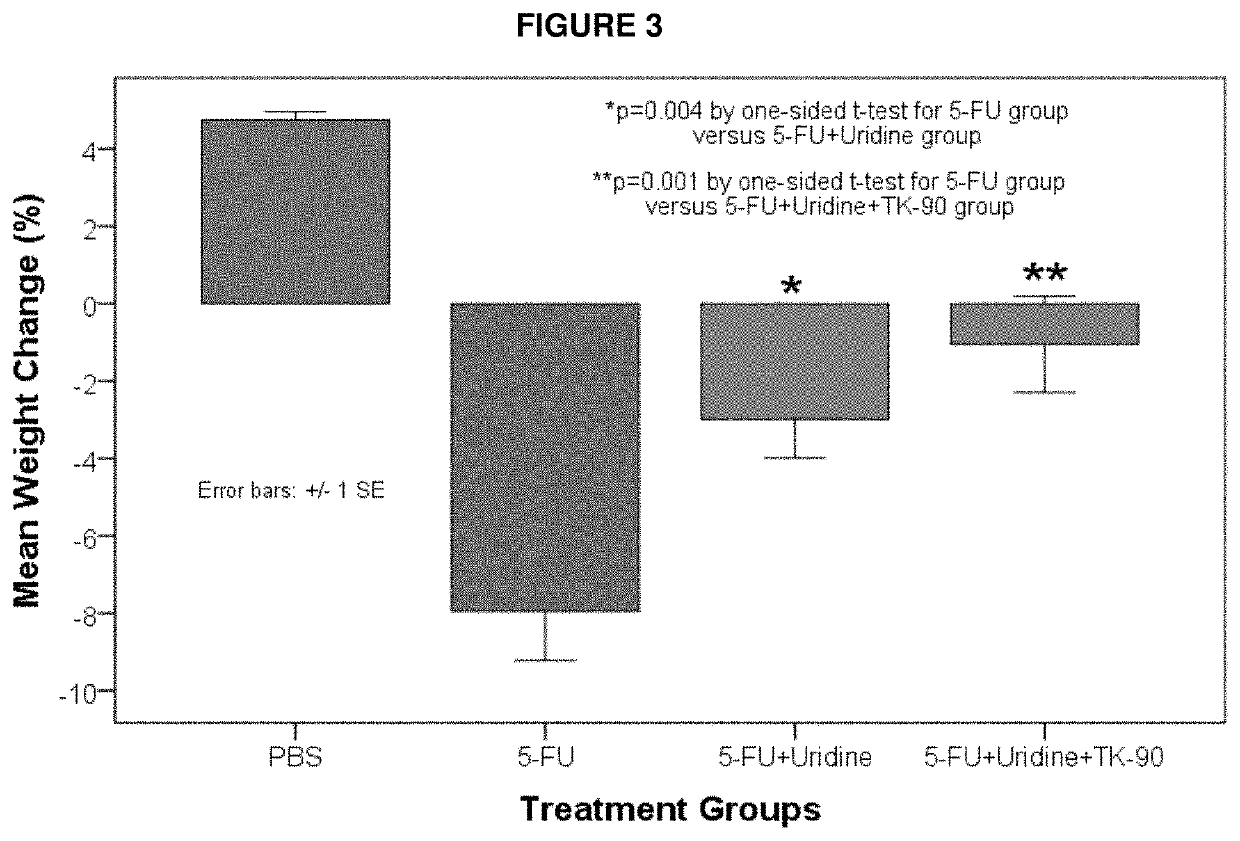
Methods and Compositions for Maximizing the Prevention and/or Reduction of Various Maladies and Drug Side-Effects Using Uridine
PendingUS20210228584A1Maximize the effect of treatmentLow toxicityOrganic active ingredientsDigestive systemDiseaseAdjuvant
Methods for improving the therapeutic effectiveness of uridine administered to prevent and / or reduce various maladies including adverse event from drugs are provided. Aspects of the methods include administering an effective amount of a uridine maximizing adjuvant, e.g., a 2,2′-anhydropyrimidine, or a derivative thereof, to the subject. Also provided are compositions for use in practicing the subject methods. The subject methods and compositions find use in a variety of different applications, including the treatment of a variety of different disease conditions.
Owner:TOSK INC
Method of mitigating adverse drug events using omega-3 fatty acids as a parenteral therapeutic drug vehicle
Owner:STABLE SOLUTIONS
Equivalent signal mining method for adverse reaction signals of srs combined medication
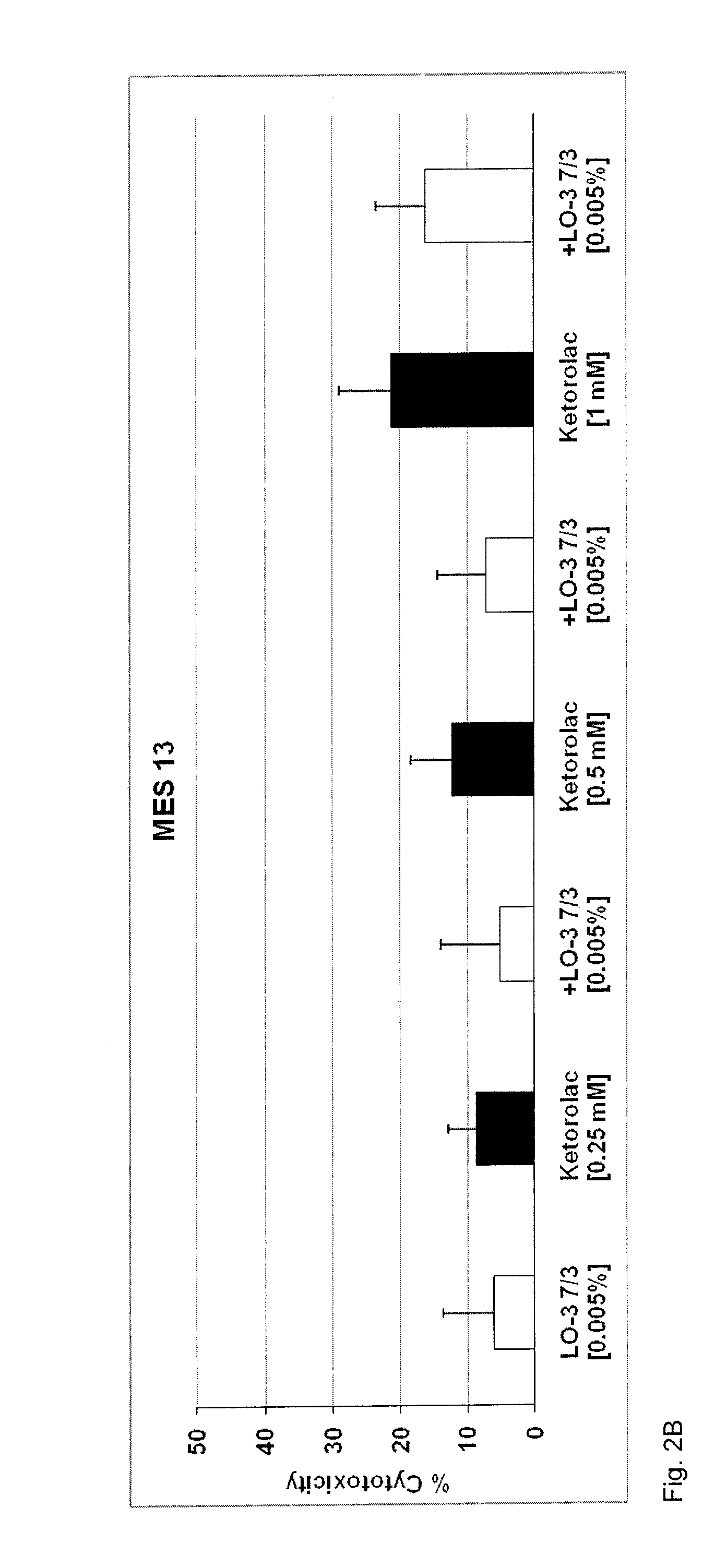
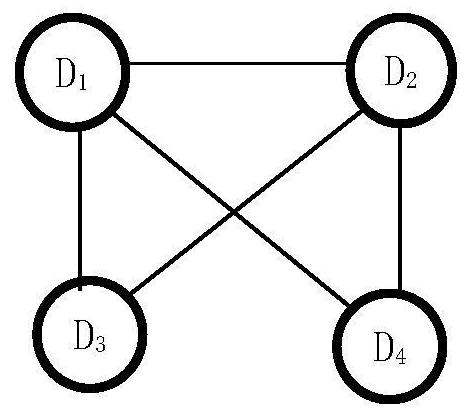
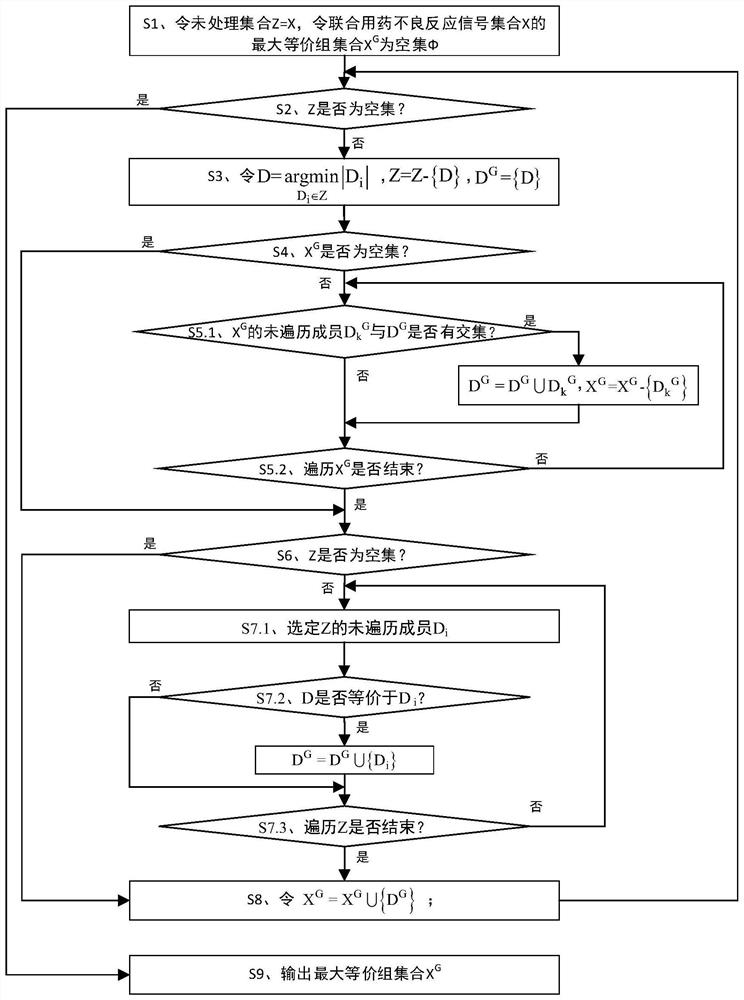
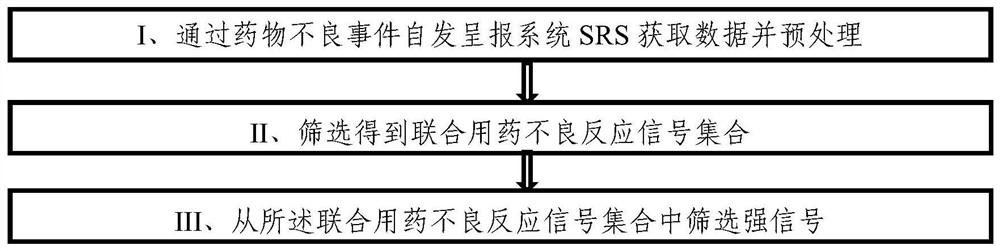
ActiveCN112365989BReduce workloadImprove analysis qualityMedical data miningDrug referencesPharmaceutical drugEngineering
The invention discloses an equivalent signal mining method oriented to SRS combined drug adverse reaction signals, including acquiring data through the spontaneous reporting system SRS of drug adverse events, and obtaining the combined drug adverse reaction signal set X={D 1 ,D 2 ,...,D x},D x To determine the drug combination as an adverse reaction signal; give priority to the signal with the smallest size of the drug combination set, and solve the largest equivalence group D of D G , combined with D G The largest equivalence group with intersection to the largest equivalence group D G ; then will not belong to D G And the signal equivalent to D is merged into D G , the D G Join X G , output the largest set of equivalence groups X G . x G Each maximum equivalence group in is the signal set with pairwise equivalence relationship between the adverse reaction signals of combined drug use. Select a signal from the maximum equivalence group to represent the signal set (that is, the maximum equivalence group), and participate in other data Analysis is beneficial to reduce the workload of the overall analysis.
Owner:长沙市弘源心血管健康研究院
An active monitoring and evaluation warning system for adverse drug events in hospitalized patients
ActiveCN109285590BRapid assessmentHealth-index calculationDrug referencesDrug utilisationHospitalized patients
The present invention provides an active monitoring, evaluation and warning system for adverse drug events in hospitalized patients. Before the system is built, multiple linked servers and a reporting system are set up. The reporting system transmits the patient's medication information and doctor's orders to multiple linked servers. The think tank is formed according to the set rules. After the think tank is built, it includes: task setting module, HIS module, inclusion module, exclusion module, judgment module, think tank review module, manual evaluation module, feature analysis module and risk warning module. Task setting module, HIS module, inclusion module, exclusion module, judgment module, think tank review module, manual evaluation module, characteristic analysis module and risk warning module are connected in sequence.
Owner:广州火龙果信息科技有限公司
An active surveillance and early warning system for drug-induced diseases
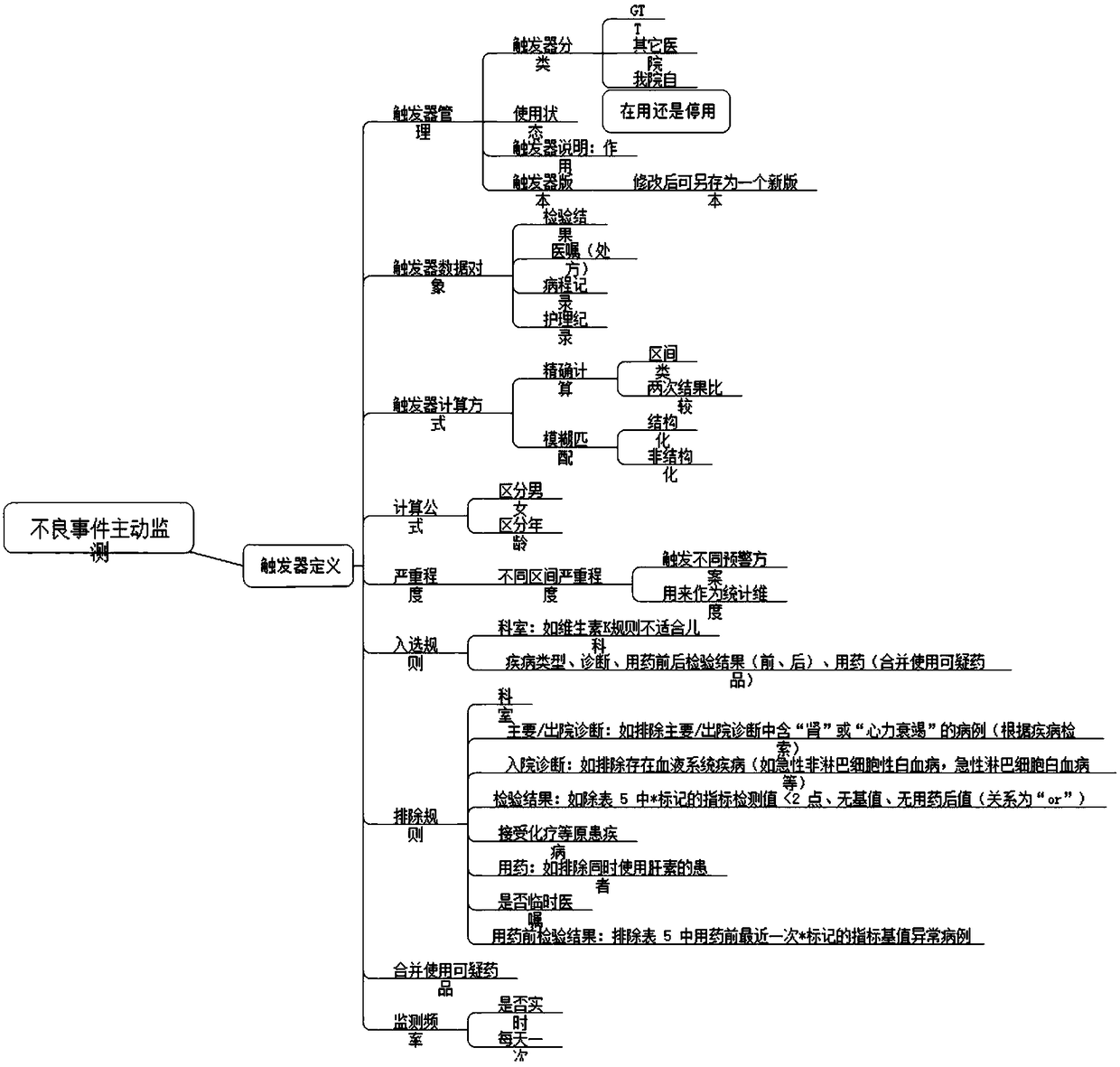
ActiveCN114005503BStrengthen the risk management and control of clinical drug useQuick searchDrug referencesAlarmsDiseaseMedication risk
Owner:SICHUAN ACADEMY OF MEDICAL SCI SICHUAN PROVINCIAL PEOPLES HOSPITAL
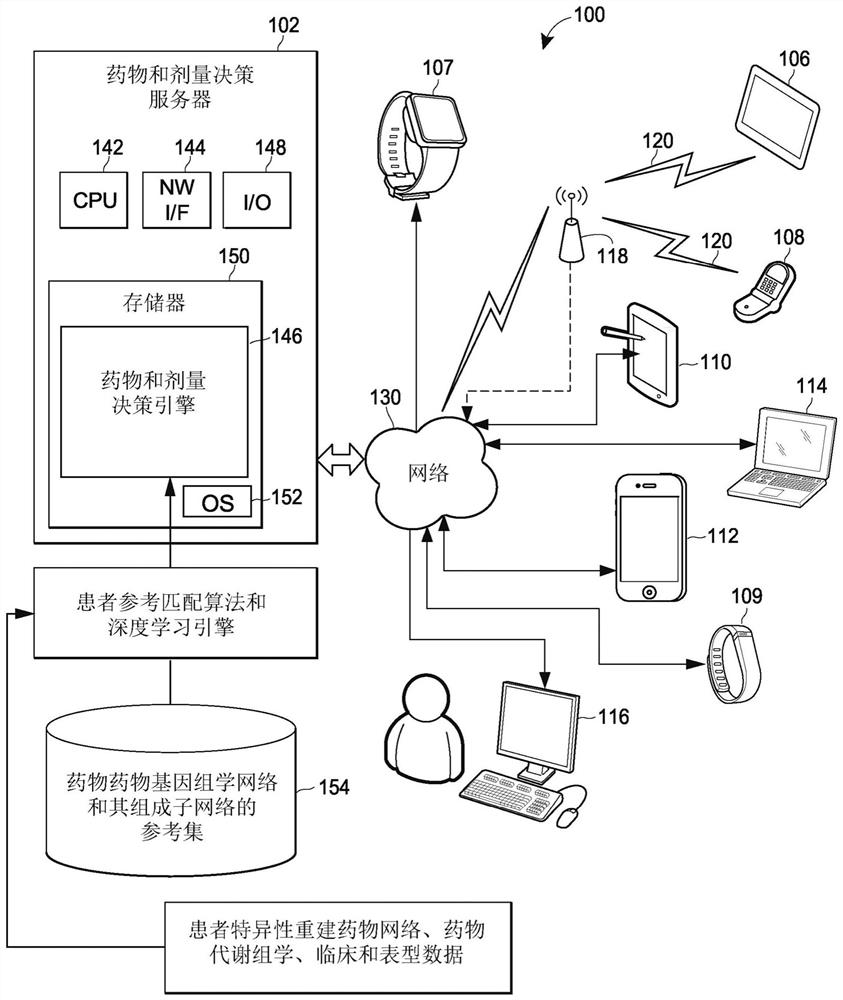
Pharmacogenomic decision support for modulators of the nmda, glycine, and ampa receptors
PendingCN113632174AExtensive computingExtensive Computing SolutionsMedical simulationDrug and medicationsNucleotideEfficacy
Methods for identifying patients diagnosed with treatment resistant or refractory depression, pain or other clinical indications who are eligible to receive N-methyl-D-aspartate receptor antagonist, glycine receptor beta (GLRB) modulator, or alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR)-based therapies to include determining the appropriate medication, an optimal dose for each patient, and determining which patients are not eligible to receive the therapy. The pharmacogenomic clinical decision support assays include targeted single nucleotide polymorphisms and clinical values or a combination of targeted single nucleotide polymorphisms, targeted ketamine- specific expansion and contraction of topologically associated domains, and clinical values. The methods described herein allow for a more effective determination of which patients will experience drug efficacy and which patients will experience adverse drug events. The methods provide personalized patient recommendations for dose, the frequency of medication administration, and recommendations on drug choice.
Owner:RGT UNIV OF MICHIGAN
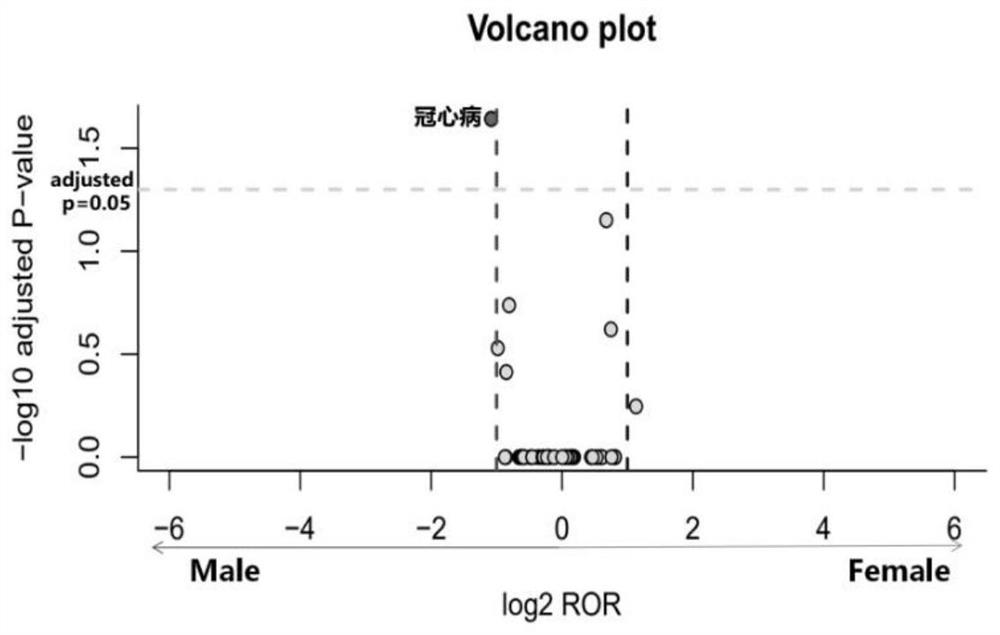
Detection method of drug sex difference adverse reaction signal
ActiveCN112185547BGender differences are well understoodProven reliabilityMedical data miningMedical automated diagnosisMale patientPharmaceutical Substances
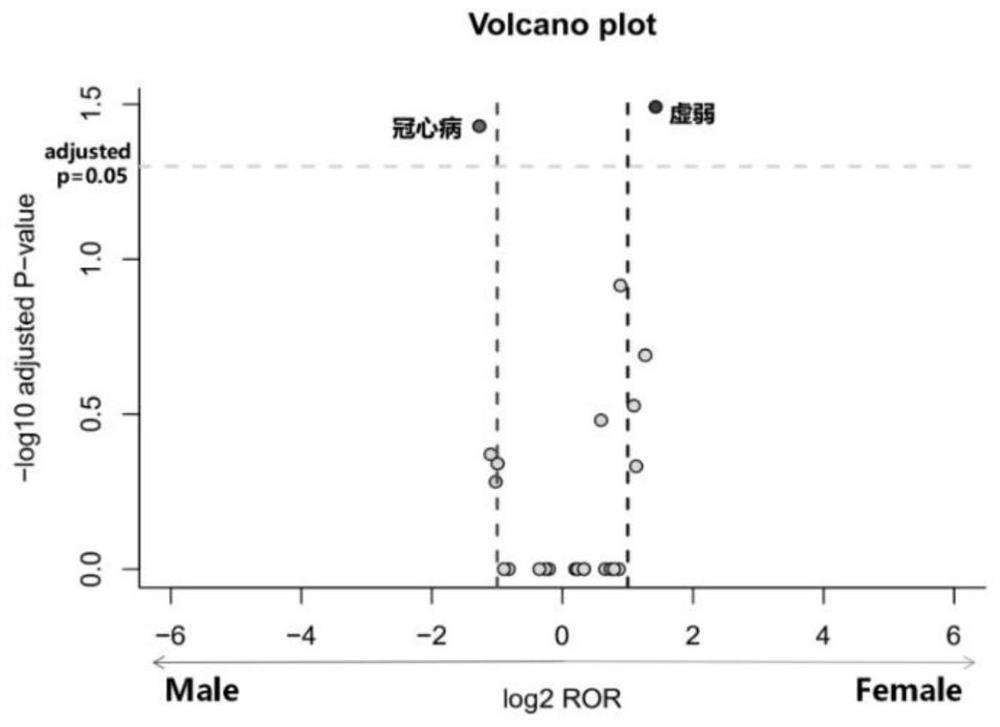
The invention discloses a detection method of a drug gender-differential adverse reaction signal: S1, data preprocessing: obtaining the drug adverse event report of the target drug from a known drug adverse event report database; S2, using the ROR algorithm to extract male and female patients The ADR signal of the target drug is calculated by dividing the ratio of the target adverse event to other adverse events in female patients with the target drug (a / b) by the ratio of the target adverse event to other adverse events in male patients with the target drug (c / d), and evaluating the occurrence of different genders The relative risk of the adverse event of the target drug; ROR value>1, women have a greater risk of the target adverse event; when 0 <ror<1则说明男性发生目标不良事件的风险更大;信号判断标准:女性目标药物‑不良事件数a>5. Number of male target drug-adverse events c>5, a+c>50; log 2 ROR>1 or log 2 ROR<‑1.
Owner:CHONGQING MEDICAL UNIVERSITY
Drug adverse event extraction method and apparatus
A method of extracting a combination of a drug and an adverse event related to the drug includes: for each of positive example combinations, negative example combinations and combinations that are neither positive examples nor negative examples, which are combinations of drug and disease, extracting medical events from medical information data about a patient and generating attribute data based on time-series information about the medical events; and learning a discriminant model based on attribute data of the positive and negative examples; and inputting attribute data corresponding to the combinations that are neither positive examples nor negative examples to the discriminant model to determine scores.
Owner:NEC CORP
Atypical antipsychotic medicinal composition
InactiveCN108926569AReduce daily dosageReduce the incidence of serious side effectsOrganic active ingredientsNervous disorderAtypical antipsychoticHigh dosage
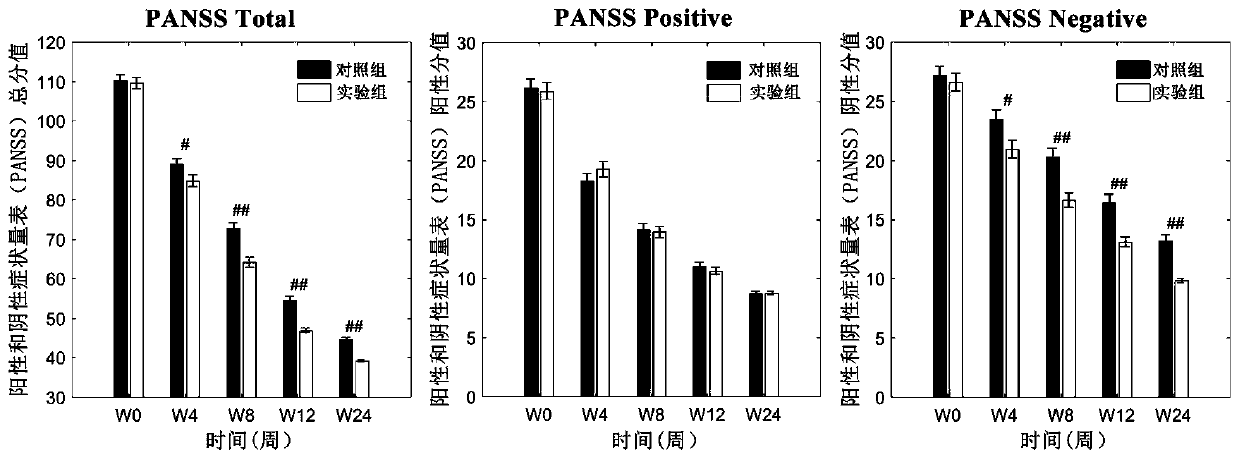
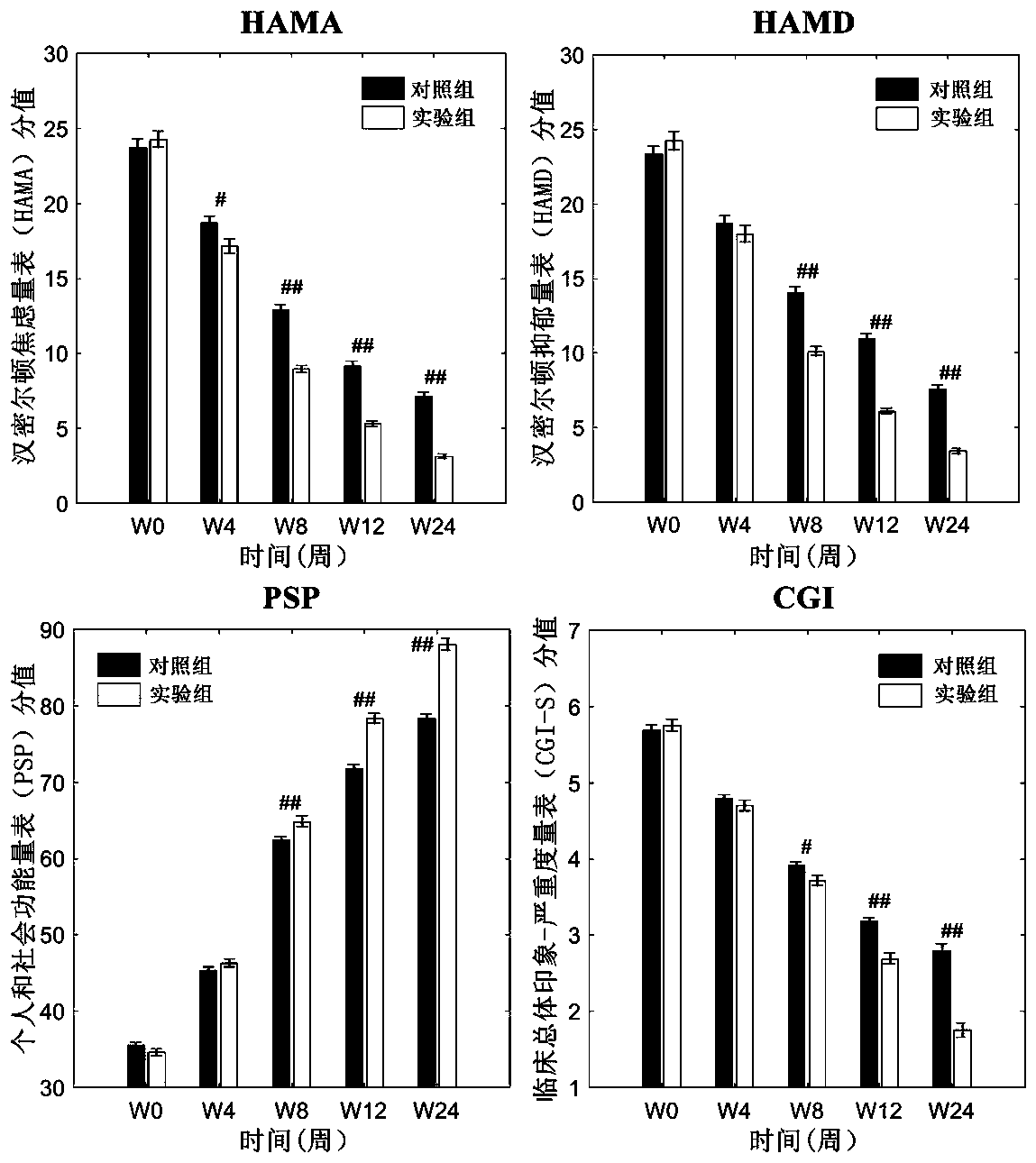
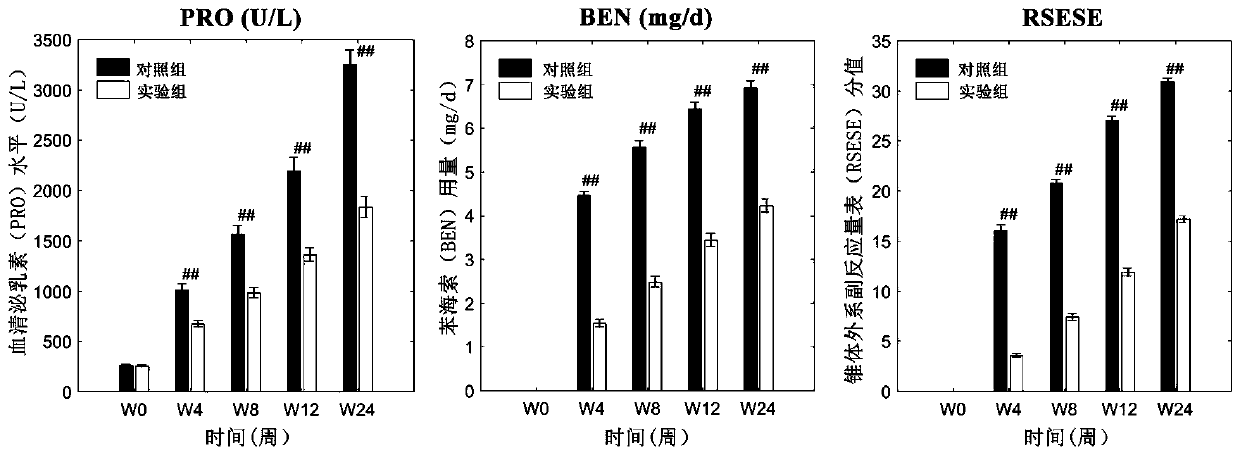
The invention relates to an atypical antipsychotic medicinal composition. The medicinal composition contains active components risperidone and sertraline hydrochloride according to a weight ratio of 0.04:1 to 0.08:1, and is applied to the treatment of SCZ when the dosage of risperidone in the active components is 2-4 mg / d. Low-dosage risperidone and sertraline hydrochloride in the medicinal composition have excellent synergistic effects; and compared with medium and high dosage risperidone, the medicinal composition has the advantages of equivalent positive symptom elimination effect, significant elimination of negative symptoms and emotion symptoms, and improvement of the social function of SCZ patients when used in the treatment of SCZ. The medicinal composition greatly reduces the occurrence probability of medium and high dosage risperidone induced hyperprolactinemia, severe extrapyramidal symptoms and other adverse drug events.
Owner:FIRST HOSPITAL OF SHANXI MEDICAL UNIV
A strong signal screening method for adverse reaction signals of SRS combination drug
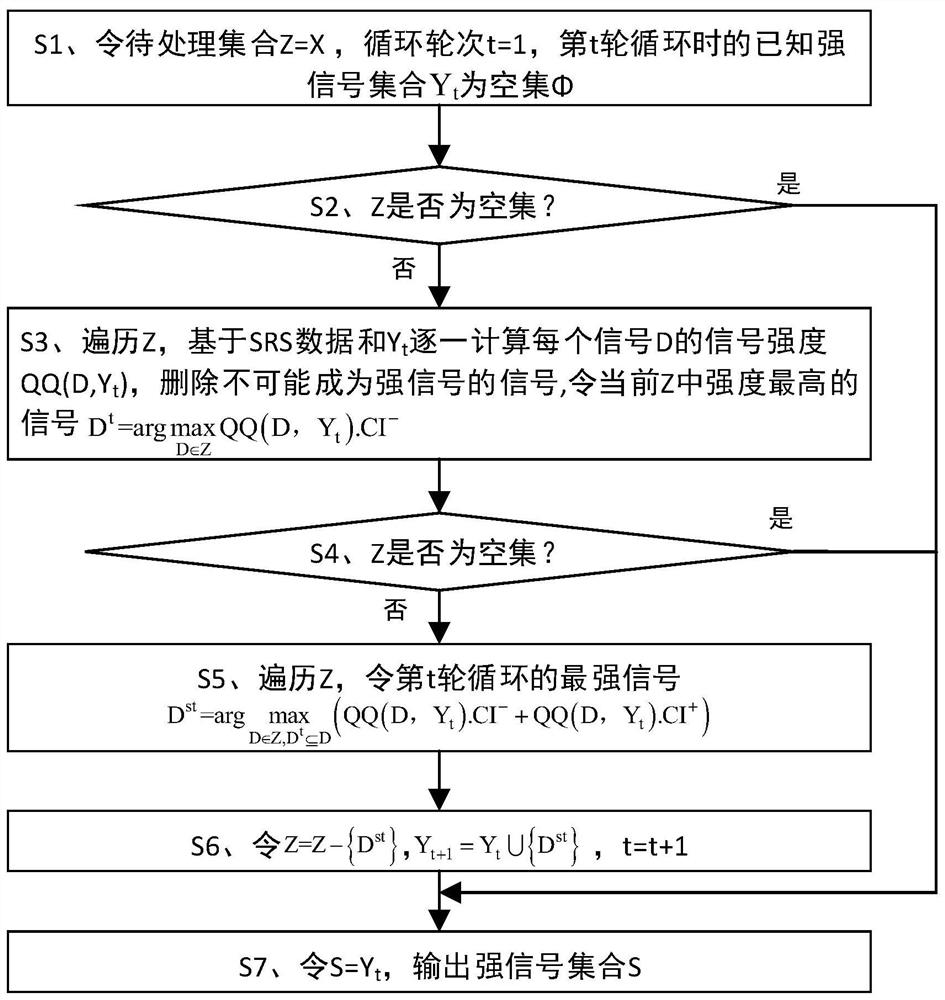
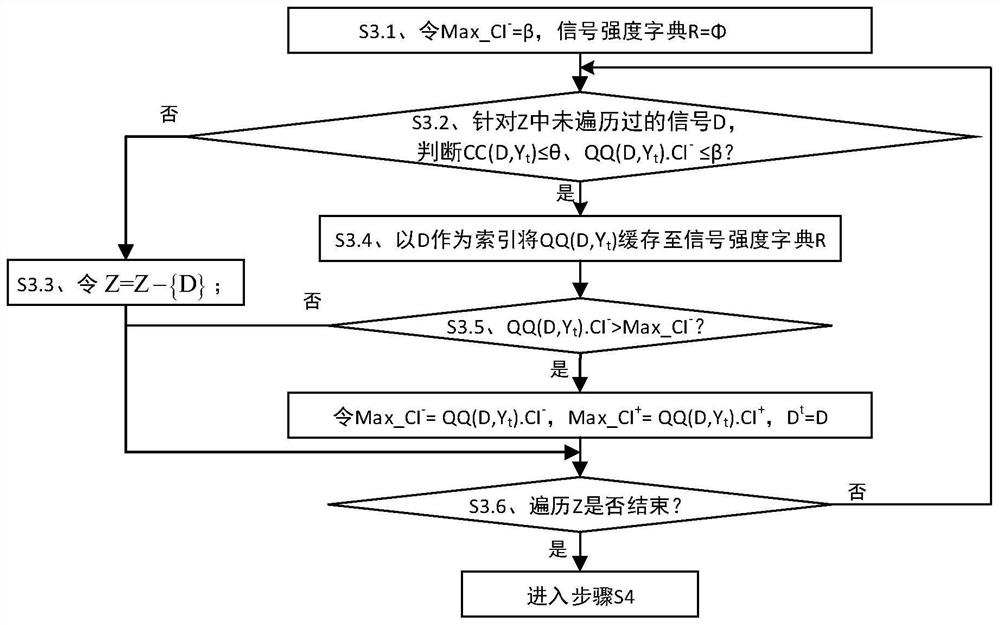
ActiveCN112365990BIncrease signal strengthImprove accuracyMedical data miningCharacter and pattern recognitionDrug utilisationPharmaceutical drug
The invention discloses a strong signal screening method for SRS combined drug adverse reaction signals, comprising: acquiring data through the spontaneous drug adverse event reporting system SRS, and obtaining combined drug adverse reaction signal set X={D 1 ,D 2 ,...,D x},D x is the drug combination judged as an adverse reaction signal; assuming that the signal exclusion set in the t-th cycle is Y t , then select the signal D with the largest current signal metric st As a strong signal, and put the signal into the signal exclusion set Y t Form a new signal exclusion set Y t+1 , and then start the t+1th round of circulation, and so on, to get the set of signals with the largest signal metric in each round of circulation, that is, the strong signal set S={D s1 ,D s2 ,...,D st}, screen out strong signals with significant case data basis from a large number of combined drug adverse reaction signals, which improves the quality of signal recommendation.
Owner:长沙市弘源心血管健康研究院
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com