Active monitoring and evaluation warning system for adverse drug events of hospitalized patients
A technology for adverse events and patients, applied in the field of electronic information, can solve problems such as speeding up treatment, failing to achieve prevention and avoiding drug safety events
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
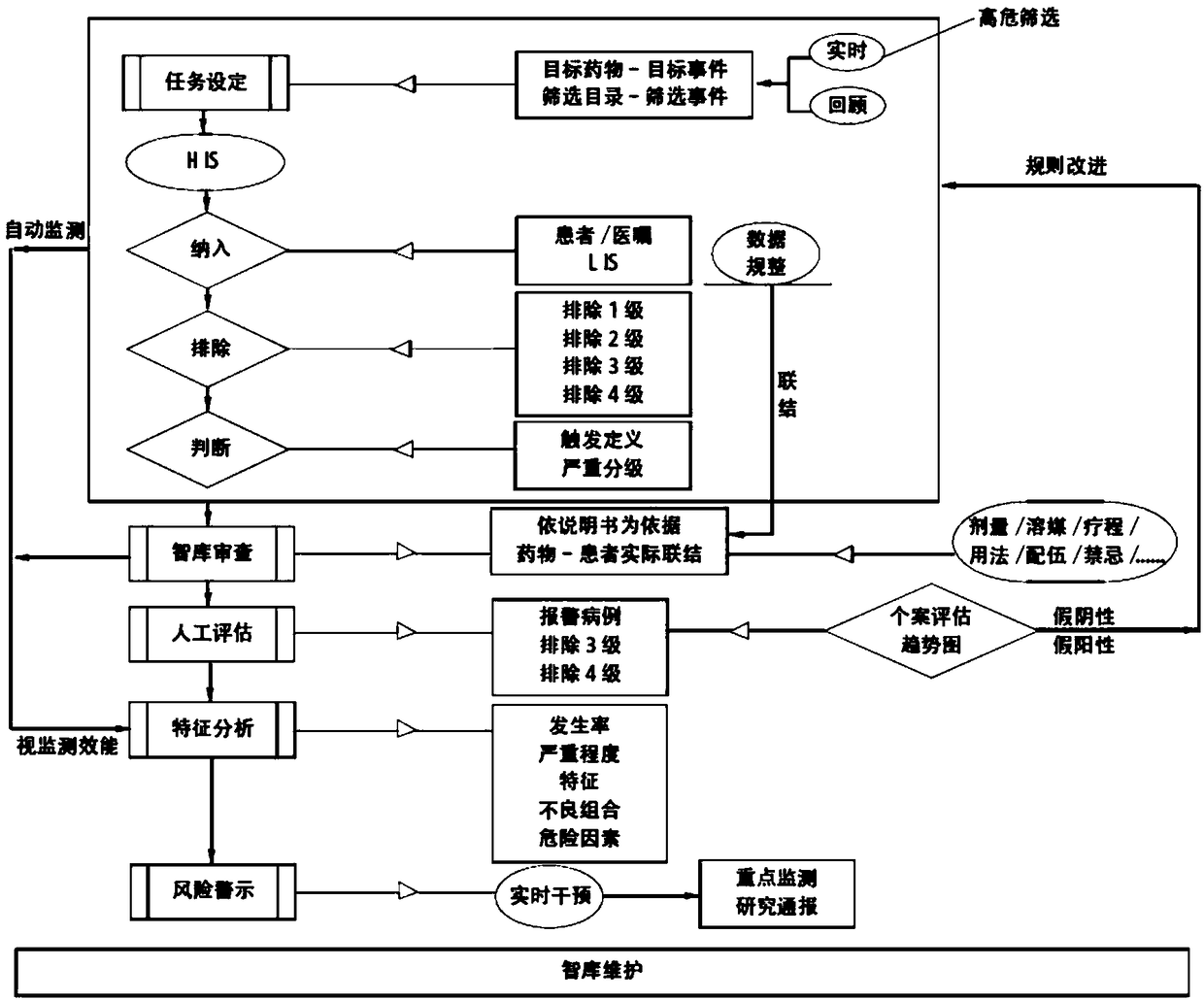
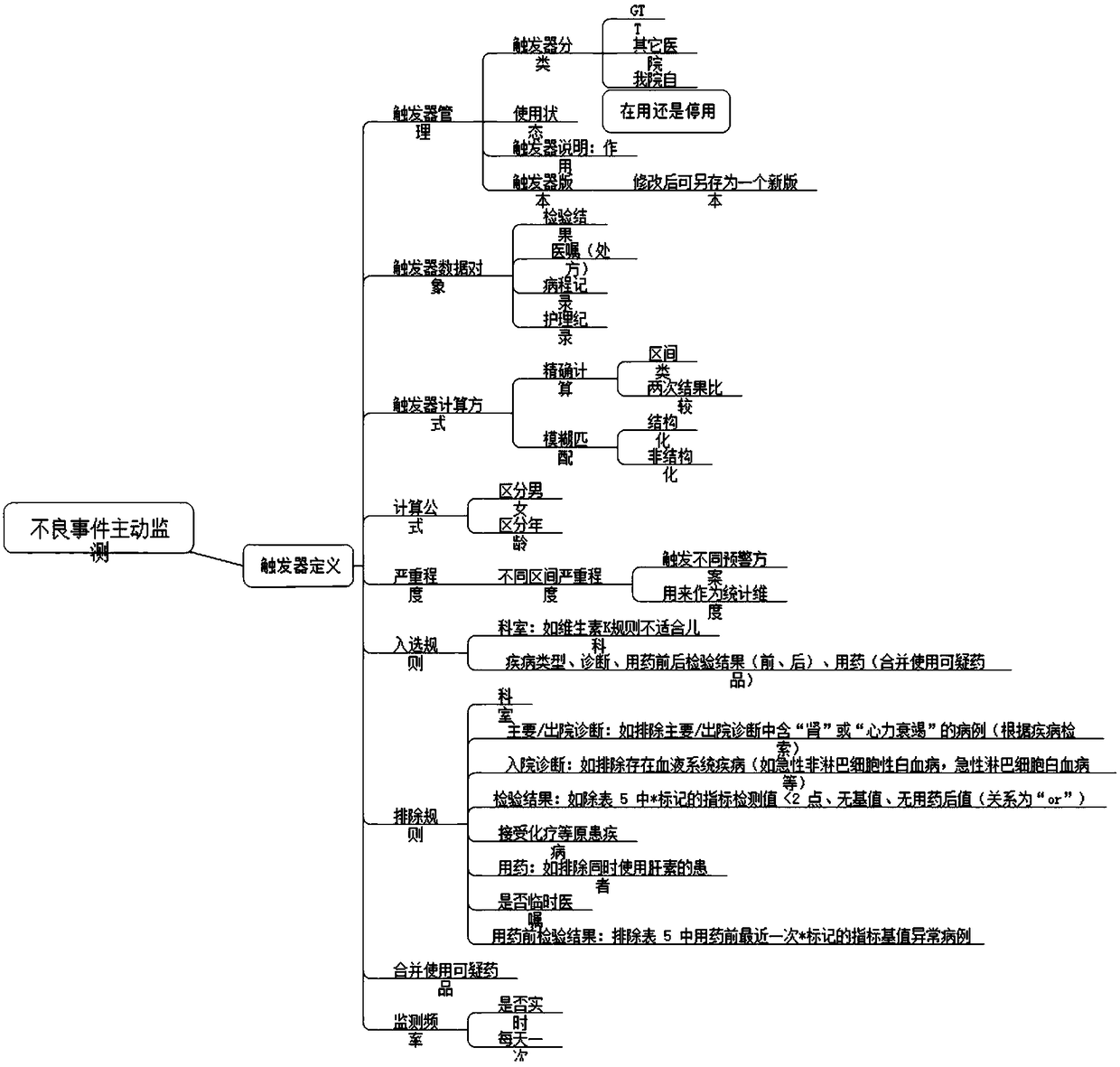
[0037] The present invention will be described in detail below in conjunction with the accompanying drawings and specific embodiments, where the schematic embodiments and descriptions of the present invention are used to explain the present invention, but not to limit the present invention.
[0038] Adverse drug events can involve respiratory, circulatory, urinary, blood, digestive, endocrine and other organ systems, and the clinical manifestations have their own characteristics. For example, there are three kinds of adverse drug reaction data sets used internationally, COSTART (Coding Symbols Thesaurus of Advesre Reaetion Terms) adverse term coding lexicon; WHO-ART (World HeathOrganization Advesre Reaetion Terms) World Health Organization adverse drug reaction data set; Med DAR ( Medical Dictionary of Drug Regulatory Affairs). Among them, my country's adverse drug reaction monitoring system is using WHO-ART, which divides adverse drug reactions into 32 categories according to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com