Patents
Literature
54 results about "Point of care device" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Point of care (POC) diagnostic devices are used to obtain diagnostic results while with the patient or close to the patient. Used in doctors’ offices, hospitals, and in patients' homes, POC diagnostic devices give quick feedback on many sorts of medical tests.
Smart medical compliance method and system
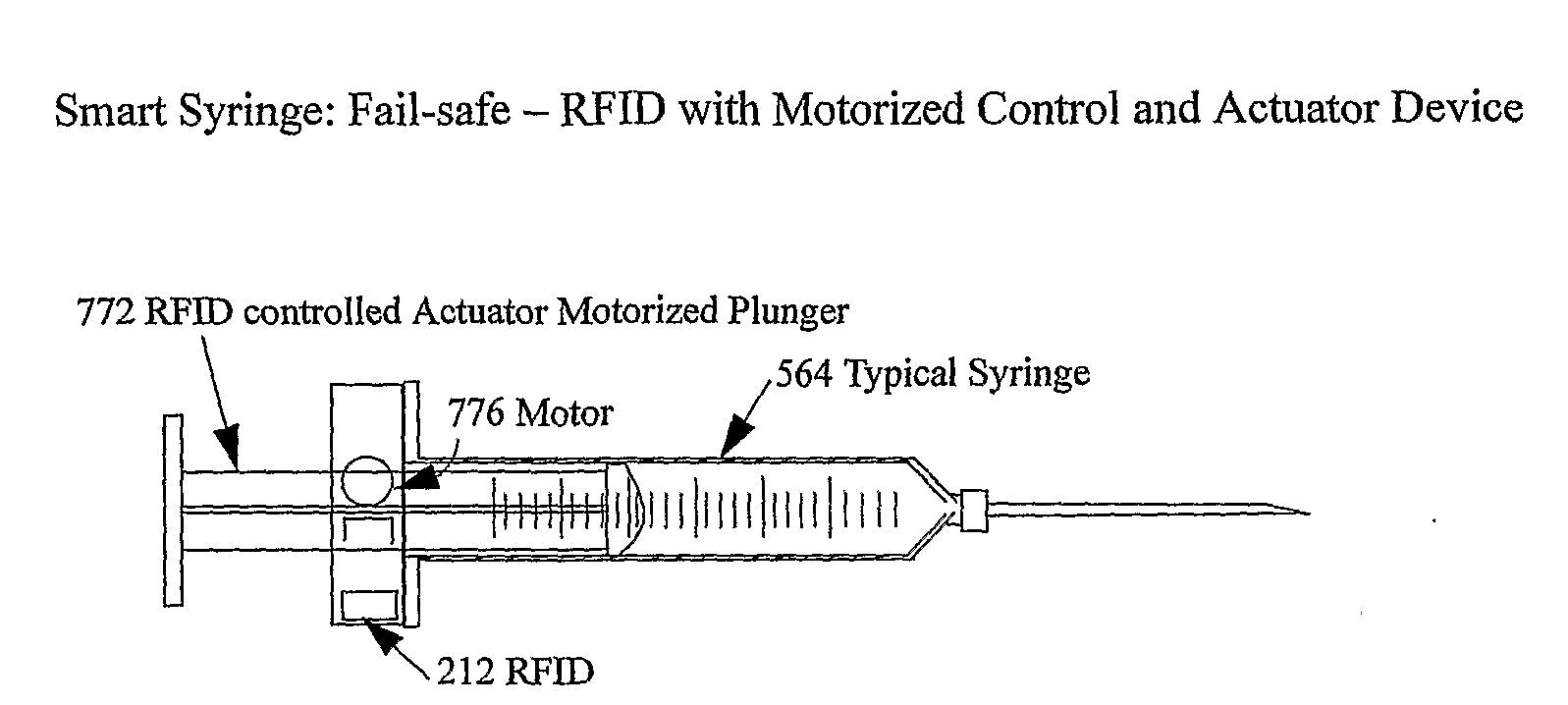
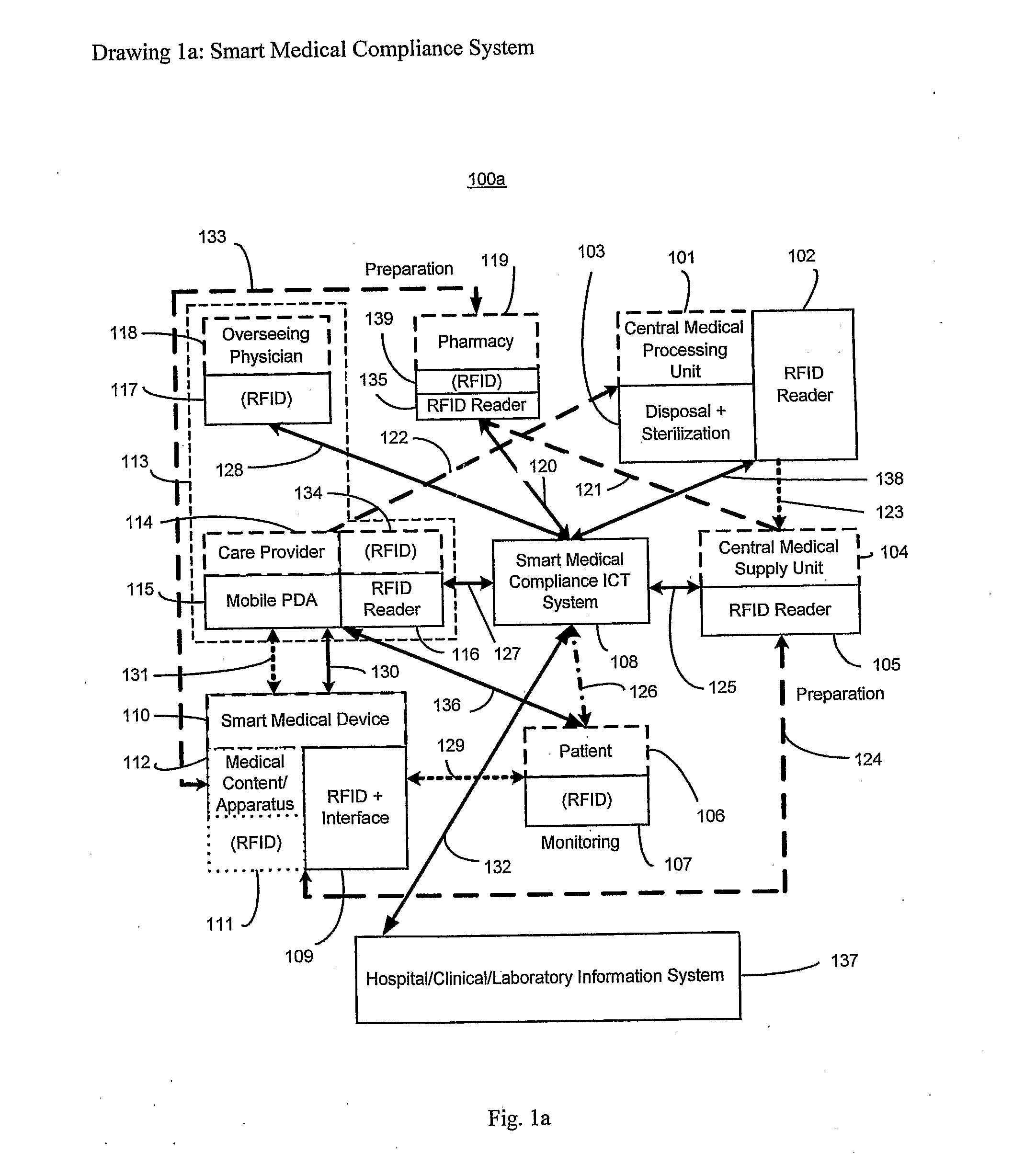
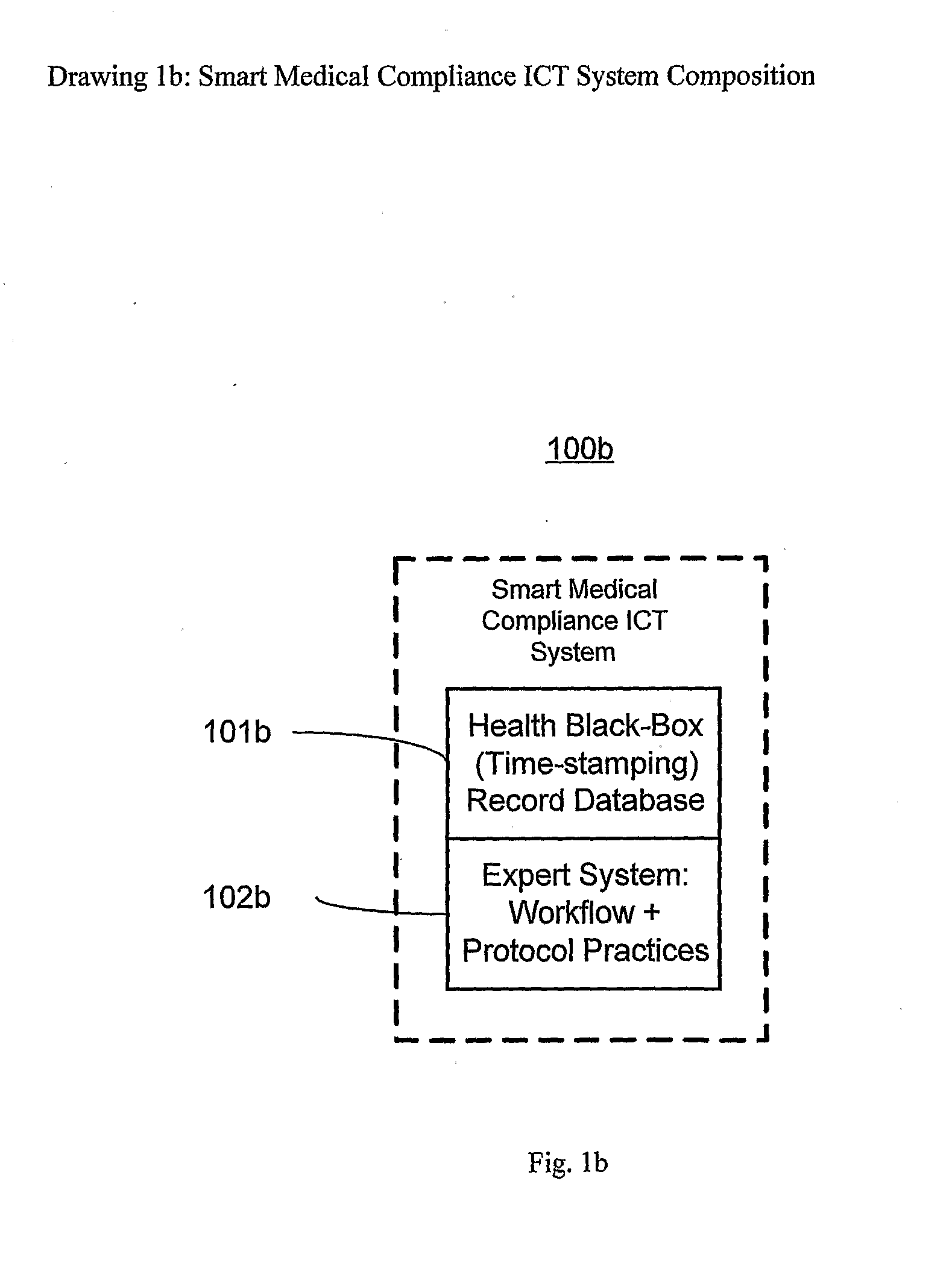
The smart medical compliance method and system invention prevents adverse drug events through the use of protocols that uniquely identifies the patient, care provider, medication and / or medical device that is to be used with radio frequency identification (RFID). The RFID devices incorporate fail-safe locks or indicators that prevent the inadvertent or unauthorized use of medication, medical devices, or medical supplies. The system corroborates, patient, the care provider, the medical device, and the manner in which it is to be used, and authorizes the action to be undertaken through an interface on a personal digital assistant PDA over a wireless communication channel. The system also timestamps events in the equivalent of a medical black box such that records may be kept to further improve patient care and allow an analysis of procedures. In addition, the system includes interfaces to medication preparation and safe disposal. A number of smart devices that interact with the system are also described. These include smart medical containers, smart clamps, smart valves, smart syringes, smart couplers, smart pipettes, and a host of other point of care devices.
Owner:KYAB LULEA
Modular point-of-care devices, systems, and uses thereof
ActiveUS20090088336A1Sequential/parallel process reactionsHeating or cooling apparatusAnalytePoint of care device
The present invention provides devices and systems for use at the point of care. The methods devices of the invention are directed toward automatic detection of analytes in a bodily fluid. The components of the device are modular to allow for flexibility and robustness of use with the disclosed methods for a variety of medical applications.
Owner:LABRADOR DIAGNOSTICS LLC
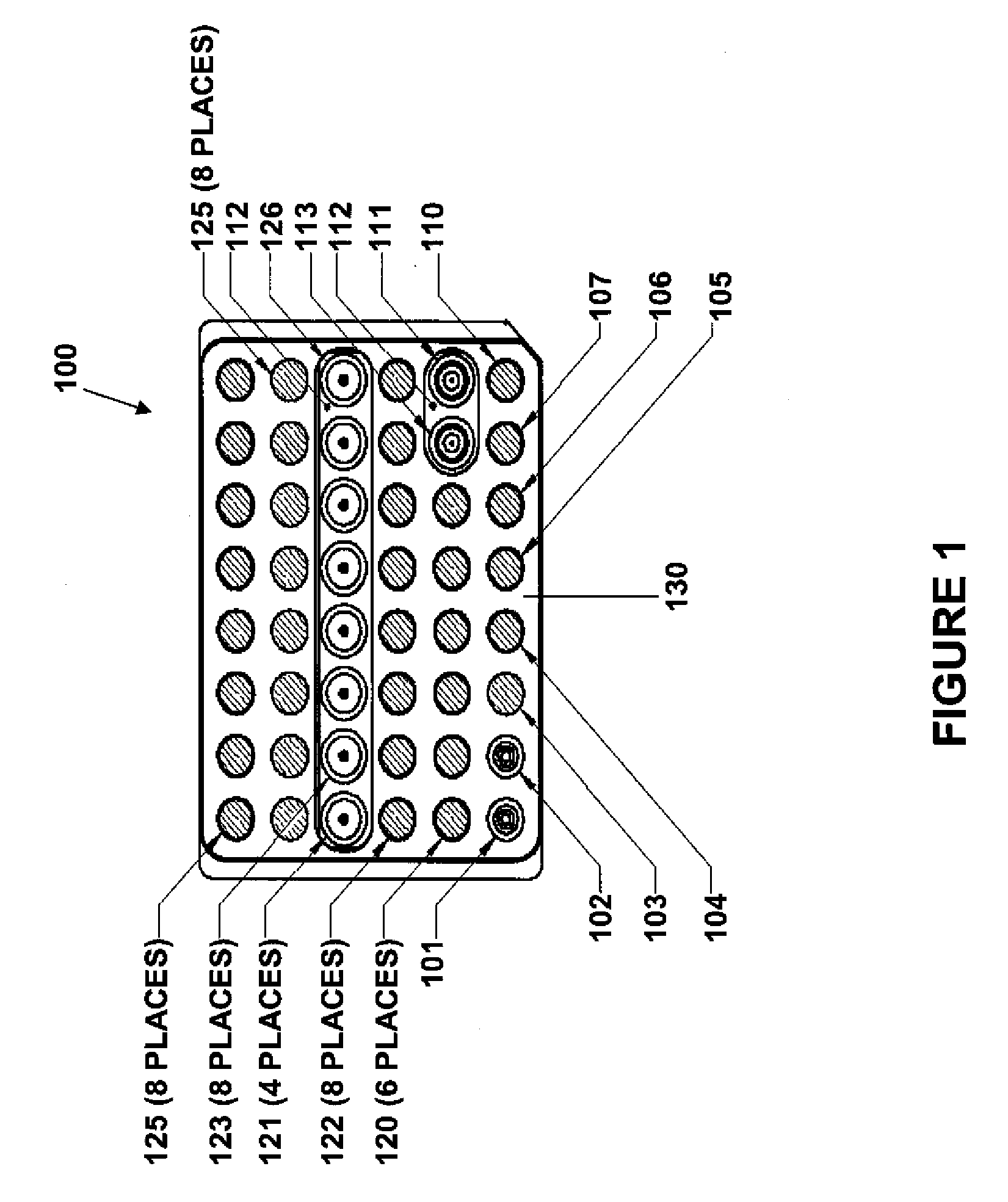
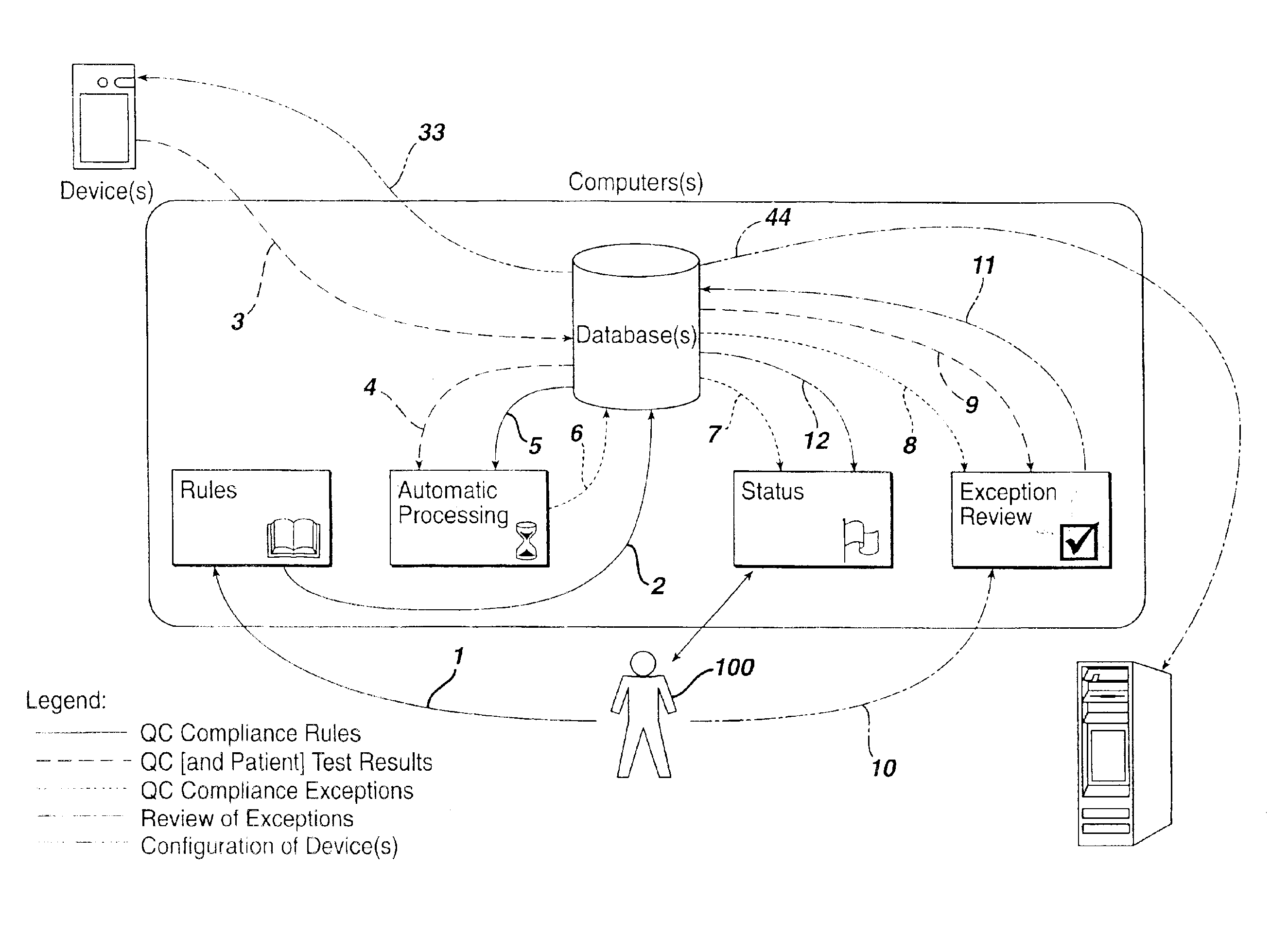
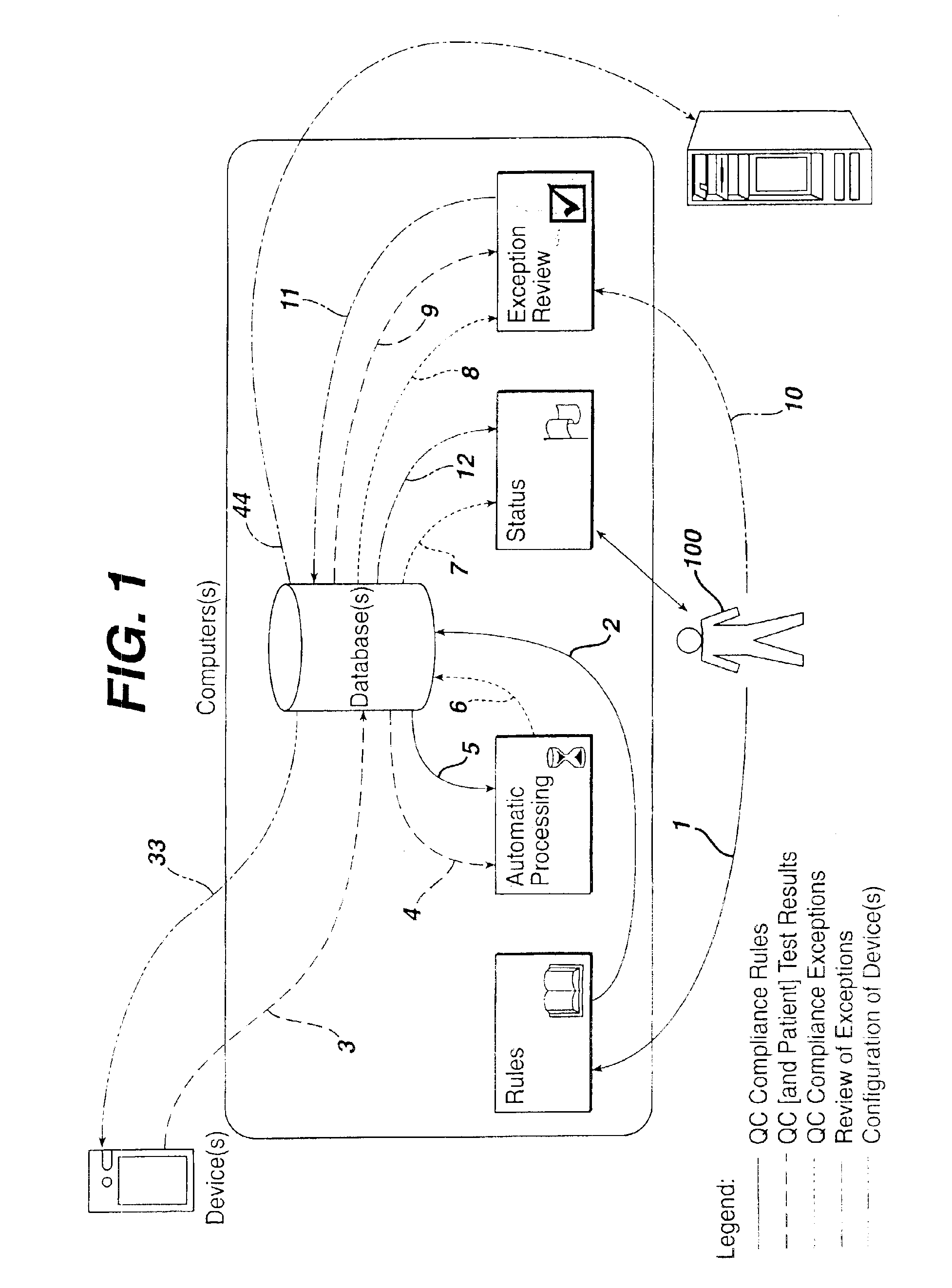
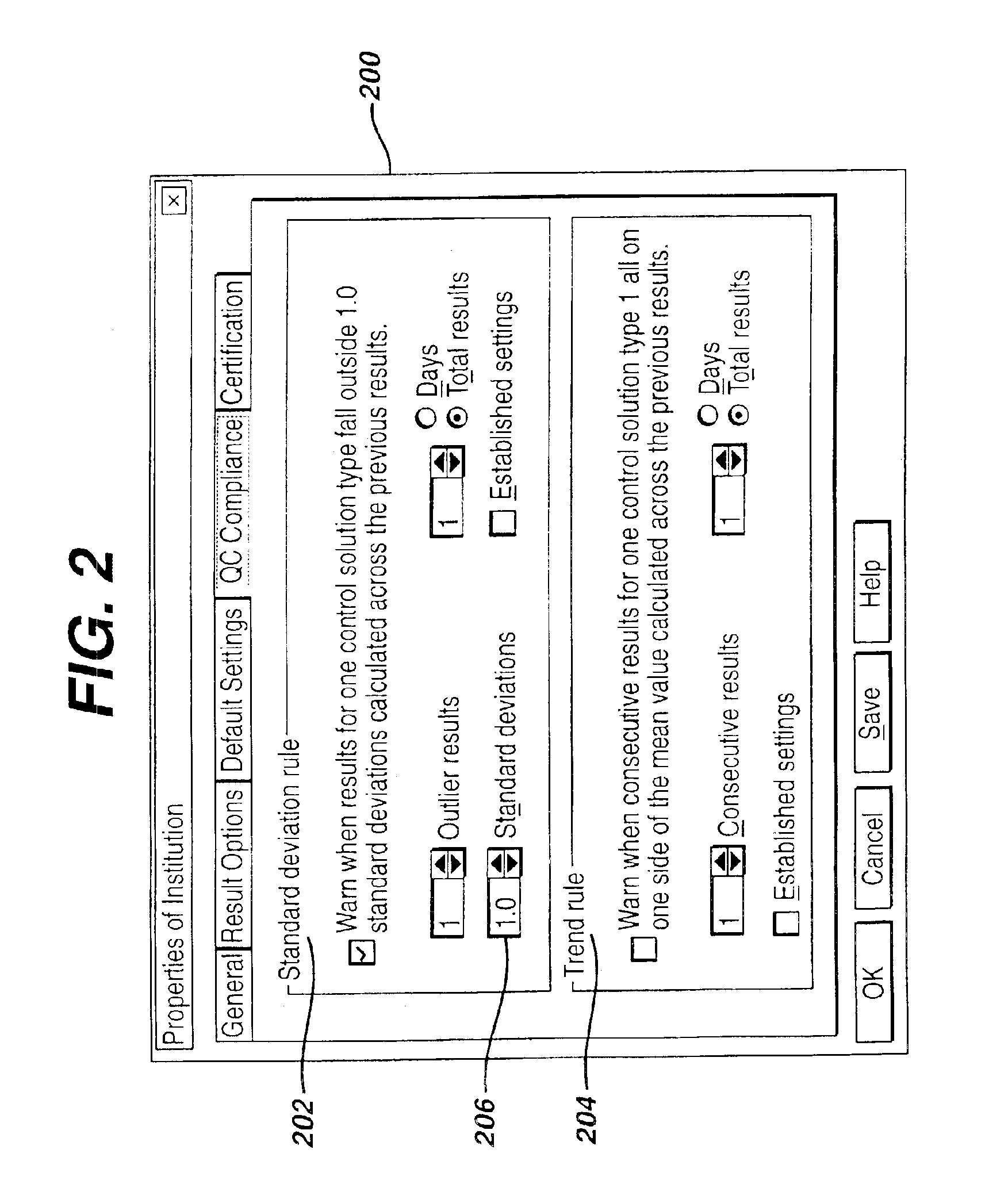
Method for automated exception-based quality control compliance for point-of-care devices
InactiveUS6856928B2Improving patient careQuality improvementDigital computer detailsNuclear monitoringAnalyteQuality control
A computer-implemented method to process POC information for potential QC compliance issues. A system and method for implementation of traditional laboratory analyzer based QC compliance in point-of-care (POC) environments is disclosed. A specific system and method to analyze data from POC testing to identify when the testing exceeds the variation expected under stable operation (i.e., the testing is “out of control”) is disclosed. This system and method is characterized by solving the QC compliance problem for POC devices by individuals not trained in traditional laboratory practices. This also provides the capability in real-time or near real-time to analyze POC testing information regarding the performance of each POC device, reagent kit (i.e., one kit per analyte tested) and / or lot, and operator so one can respond quickly to a particular device, reagent kit and / or lot, or operator that is not performing properly.
Owner:LIFESCAN IP HLDG LLC
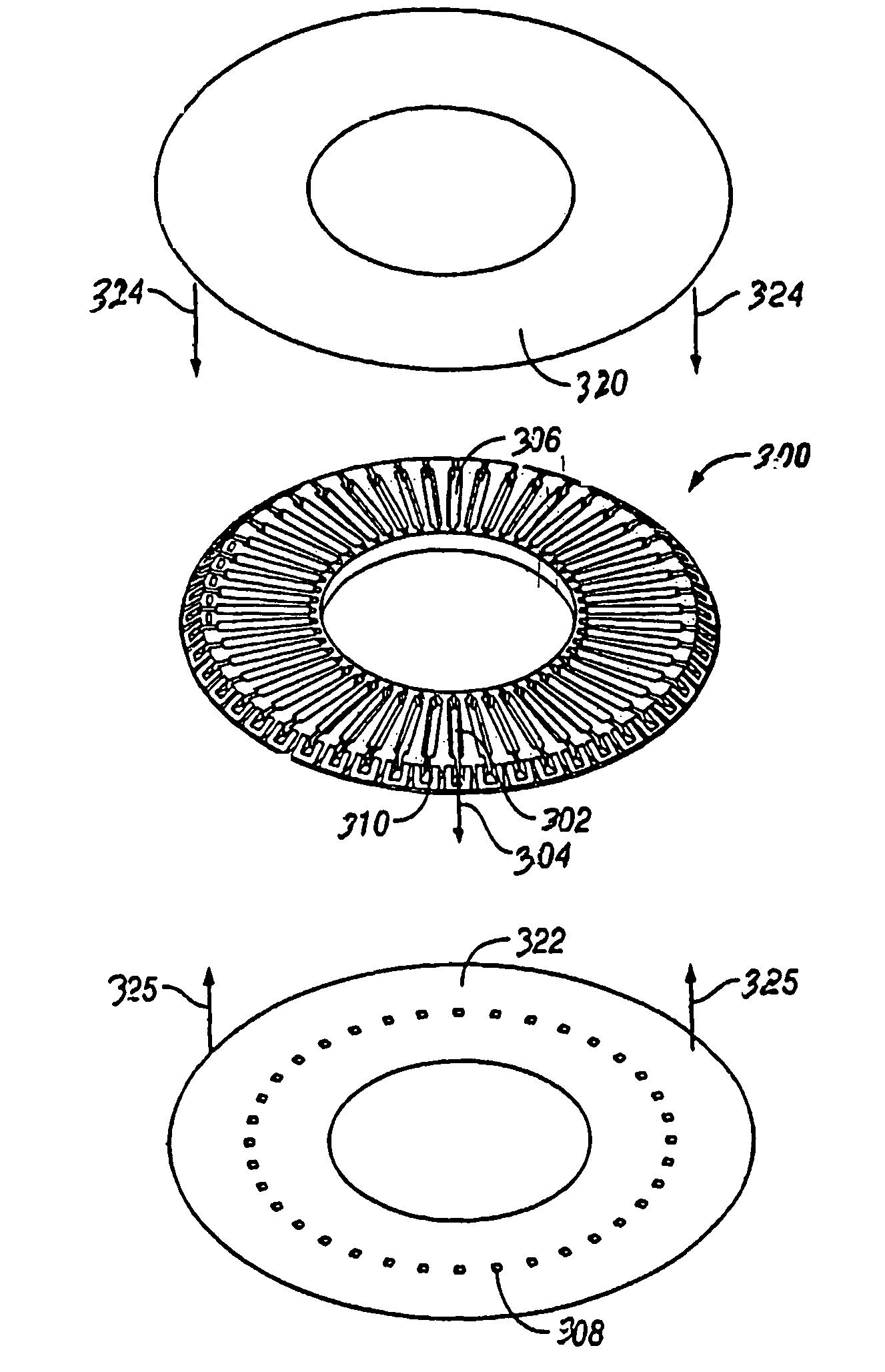
Method and apparatus for a point of care device
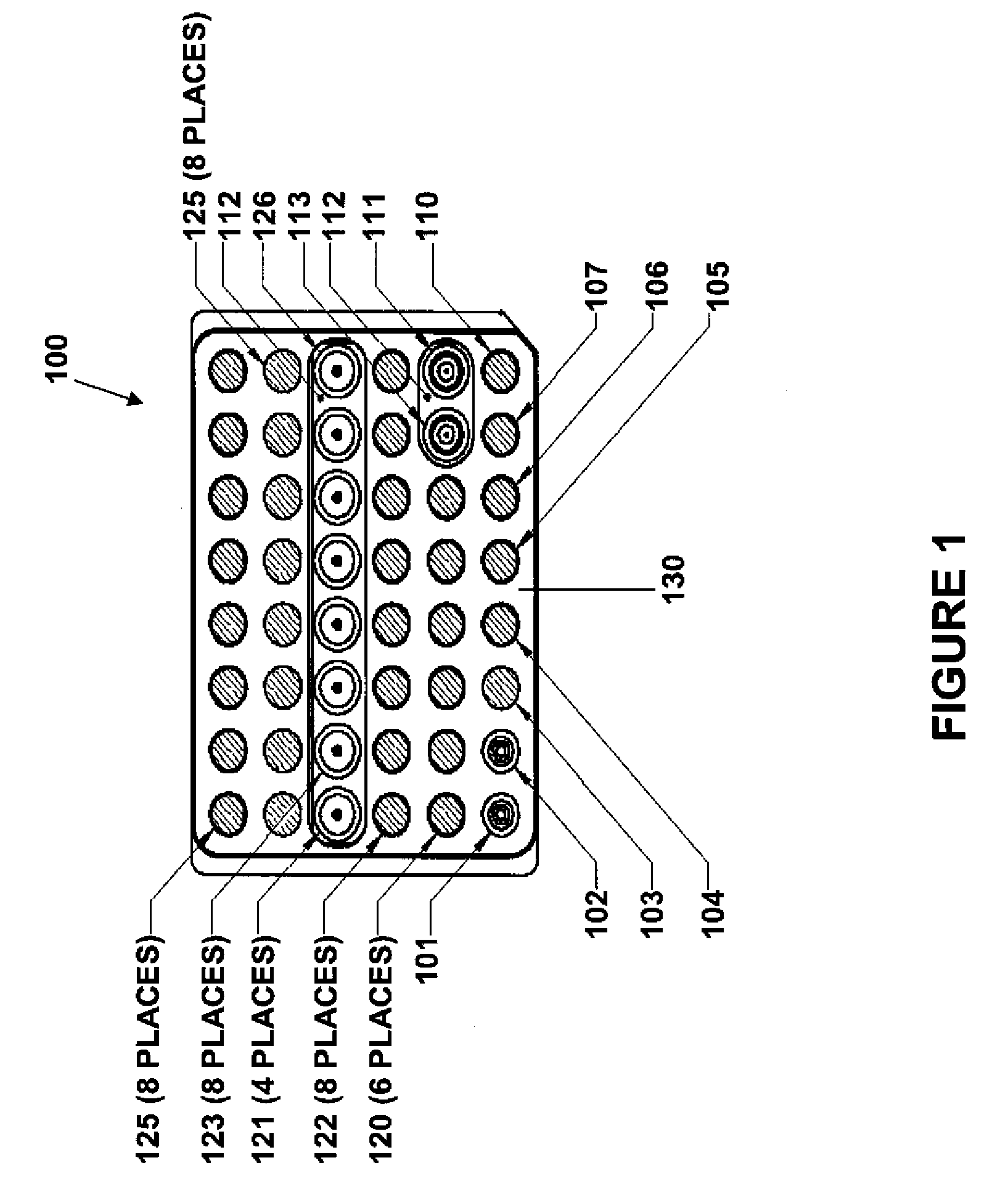
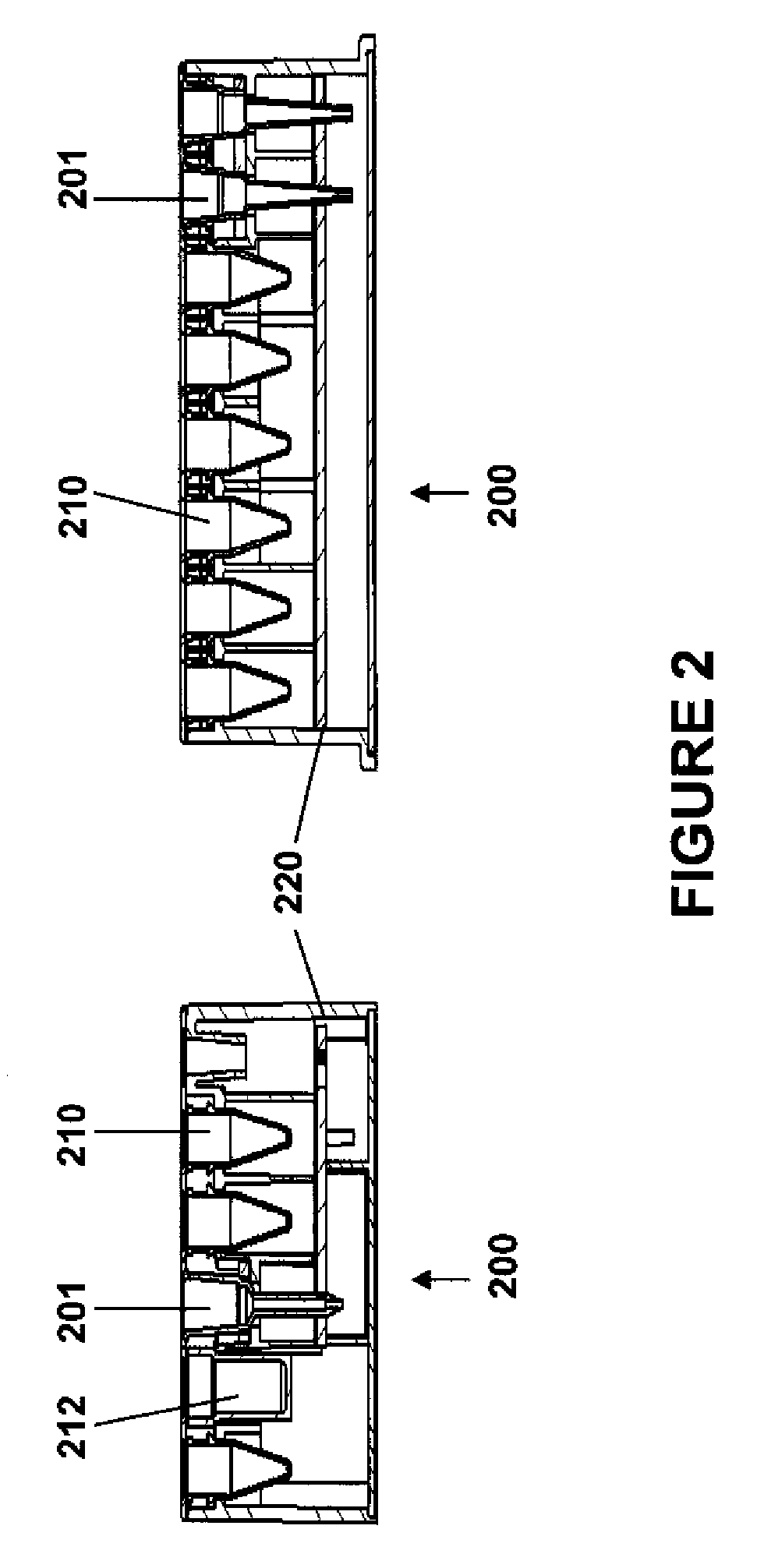
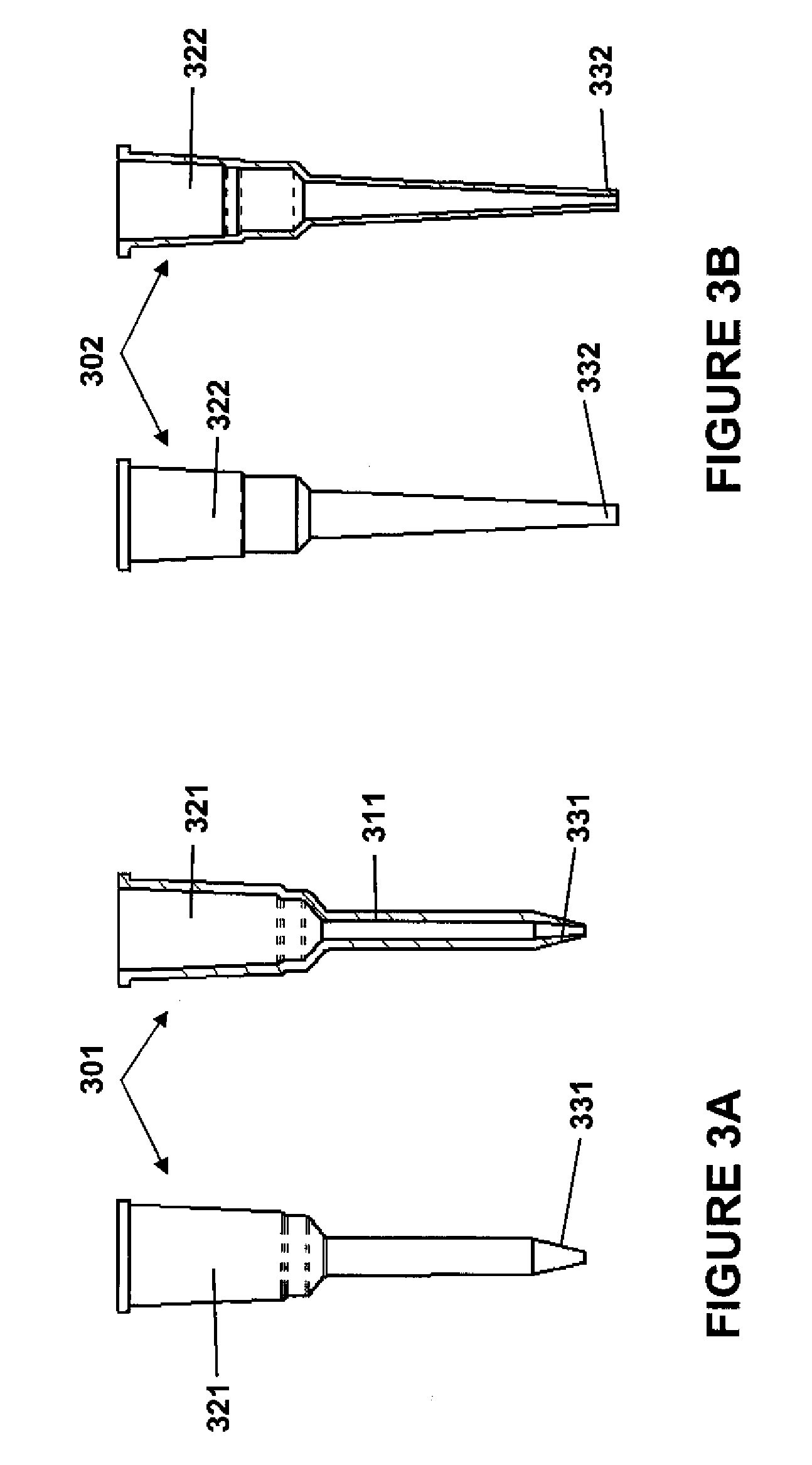
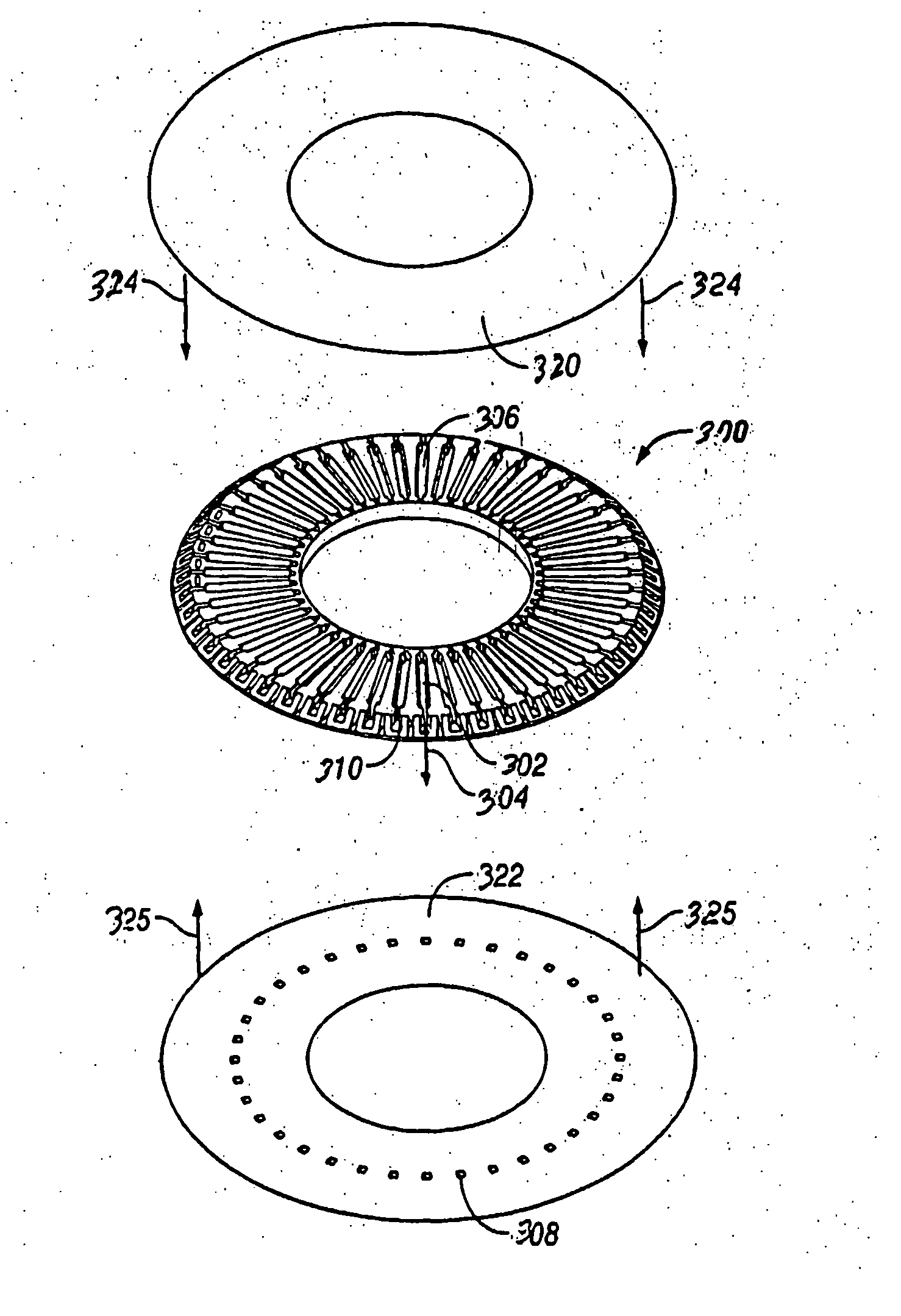
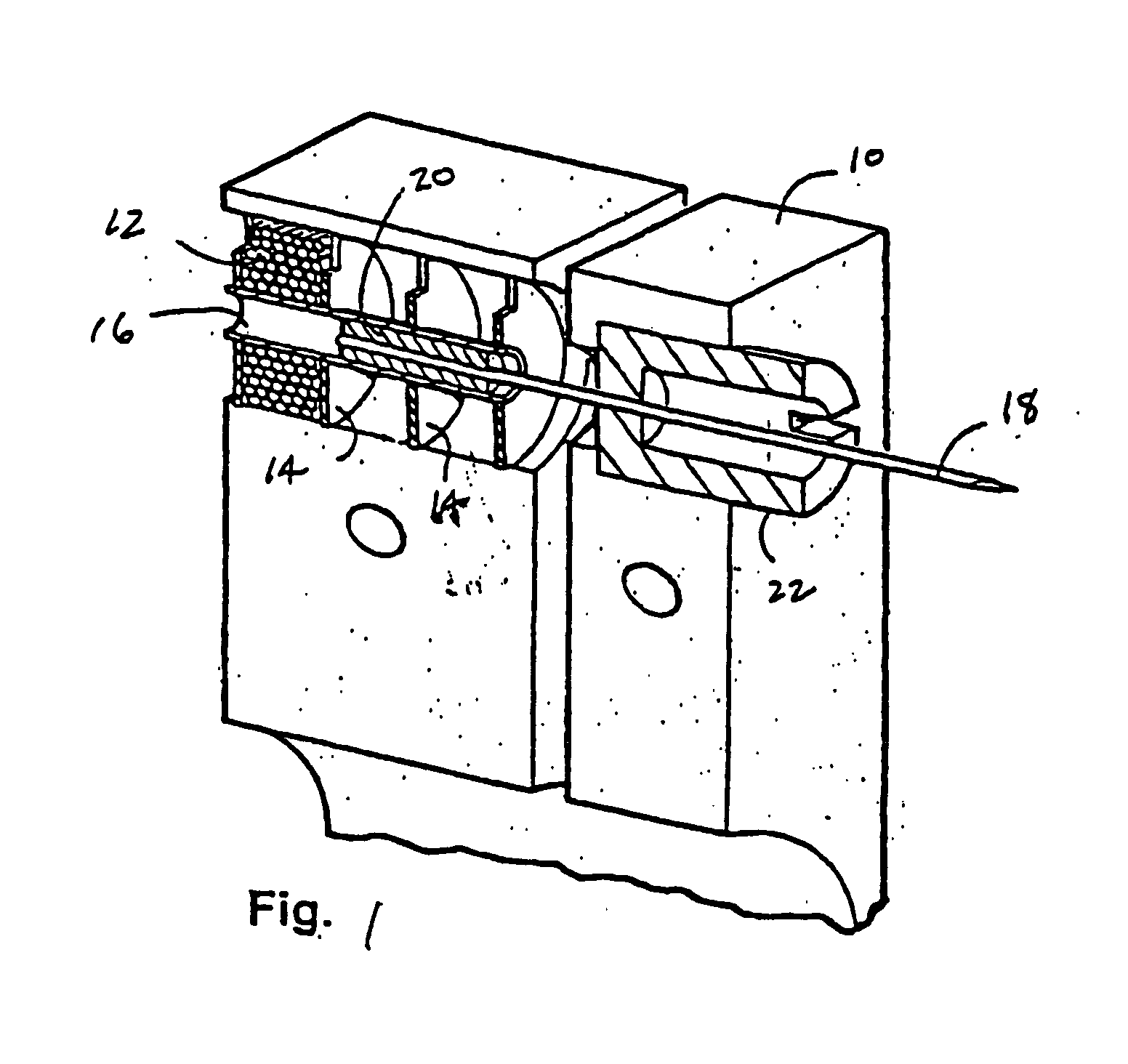
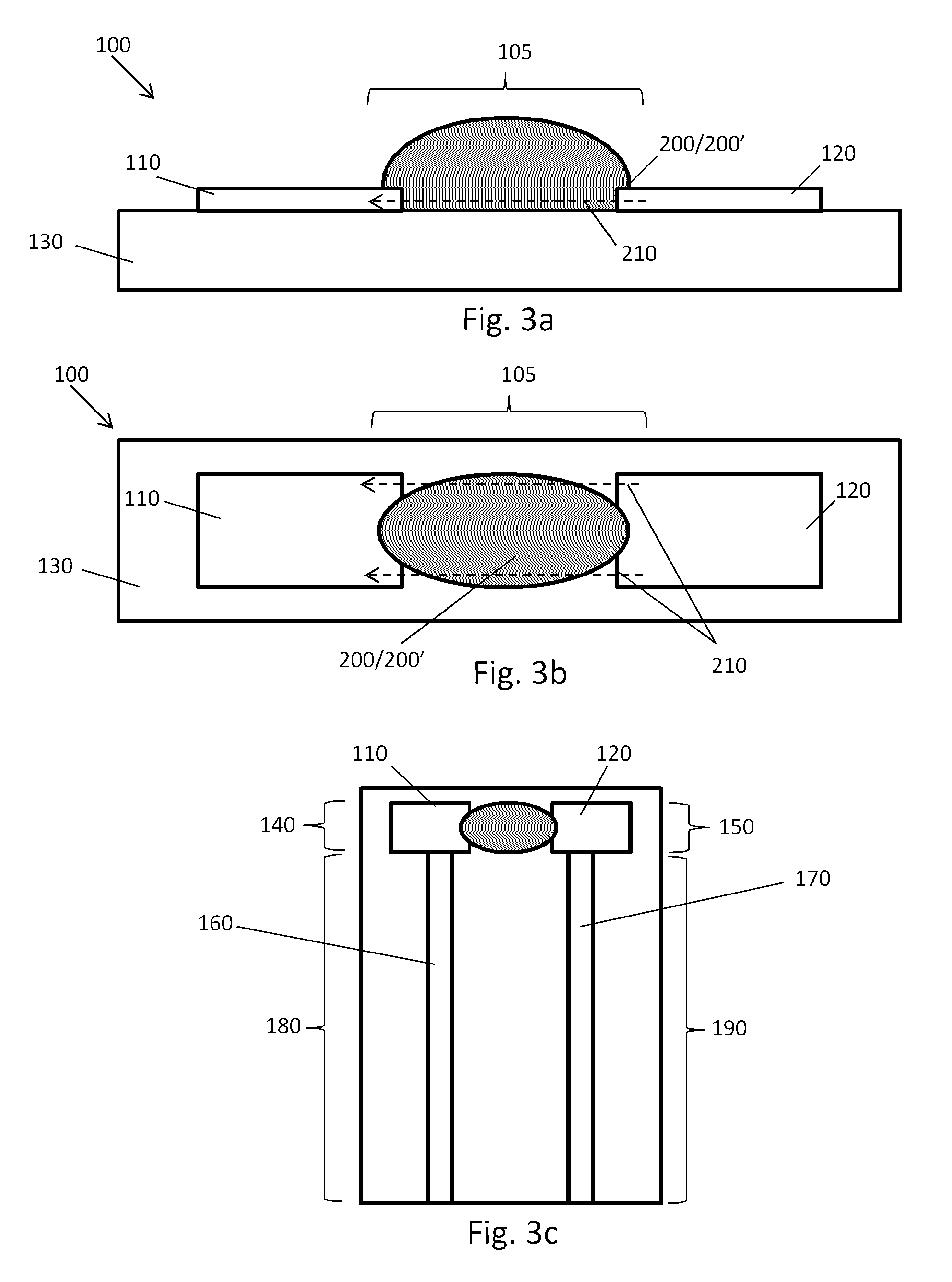
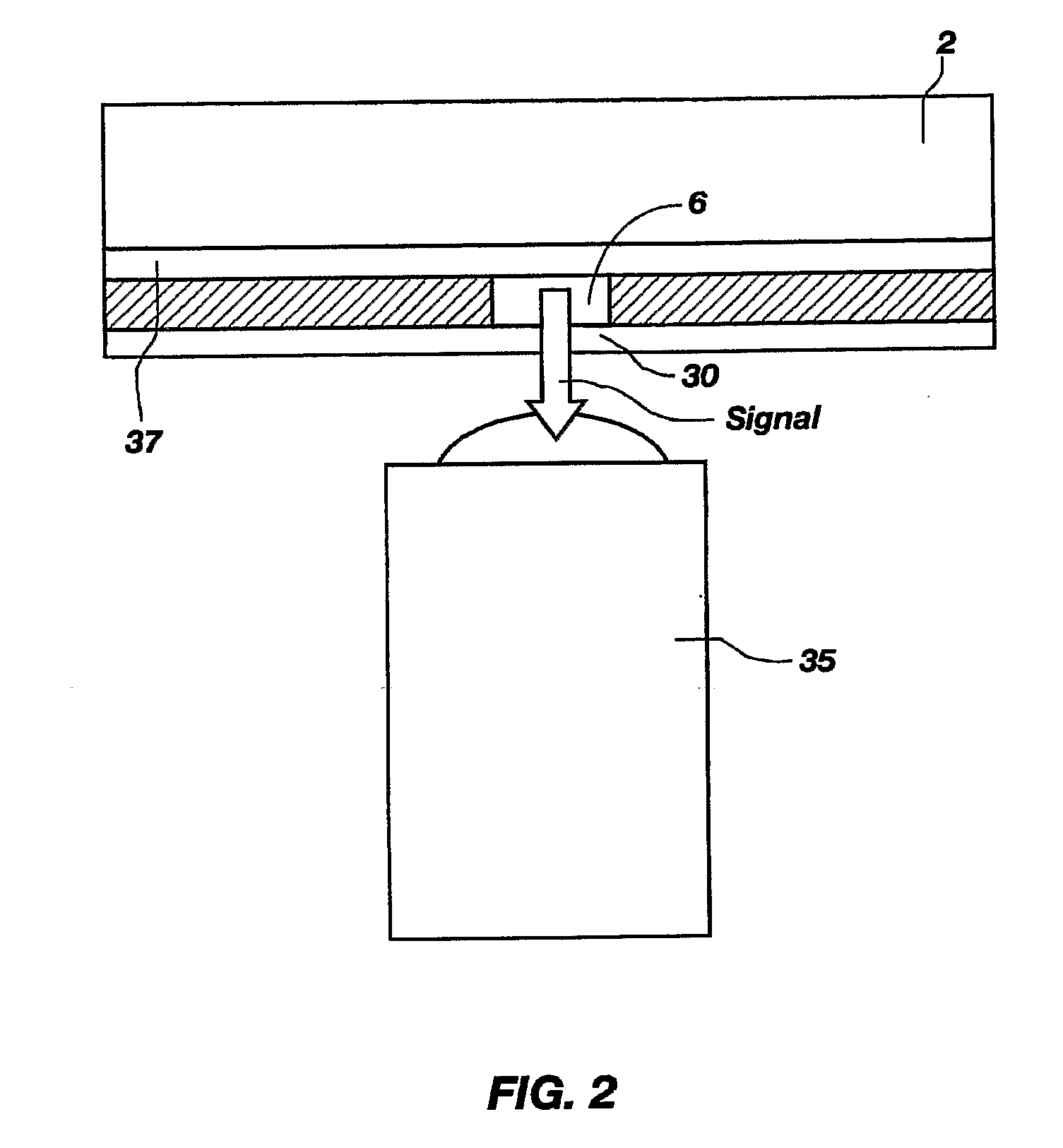
A plurality of Point-of-Care (POC) tests on a single cartridge (300) is provided such that sequential or nonsequential tests may be performed in an integrated fashion without changing the test cartridge. Each cartridge can contain a penetrating member sensor (302) combination in a radial disk format, interrogated and read by a single illumination / detection device. Alternatively a series of tests can be measured electrochemically and reported. Only those tests, which are required at the time, the sample is taken need to be reported, though all tests are carried out.
Owner:SANOFI AVENTIS DEUT GMBH
Modular point-of-care devices, systems, and uses thereof
ActiveUS8088593B2Sequential/parallel process reactionsHeating or cooling apparatusAnalytePoint of care device
The present invention provides devices and systems for use at the point of care. The methods devices of the invention are directed toward automatic detection of analytes in a bodily fluid. The components of the device are modular to allow for flexibility and robustness of use with the disclosed methods for a variety of medical applications.
Owner:LABRADOR DIAGNOSTICS LLC
Method and apparatus for a point of care device
A plurality of Point-of-Care (POC) tests on a single cartridge (300) is provided such that sequential or nonsequential tests may be performed in an integrated fashion without changing the test cartridge. Each cartridge can contain a penetrating member sensor (302) combination in a radial disk format, interrogated and read by a single illumination / detection device. Alternatively a series of tests can be measured electrochemically and reported. Only those tests, which are required at the time, the sample is taken need to be reported, though all tests are carried out.
Owner:SANOFI AVENTIS DEUT GMBH
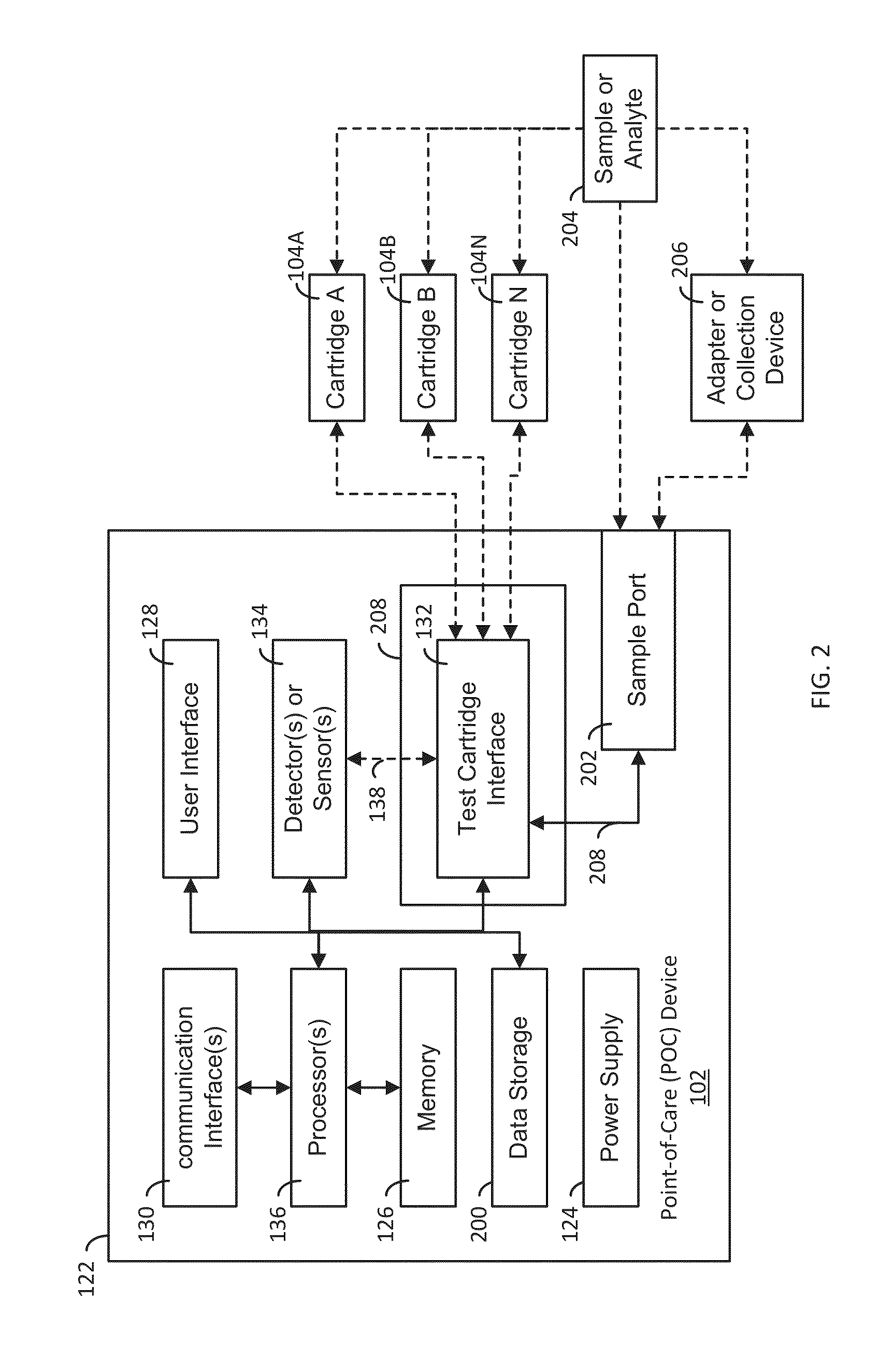
System, Apparatus and Method for Evaluating Samples or Analytes Using a Point-of-Care Device
ActiveUS20130085680A1Easy to integrateEasy to implementBioreactor/fermenter combinationsBiological substance pretreatmentsCommunication interfacePoint of care device
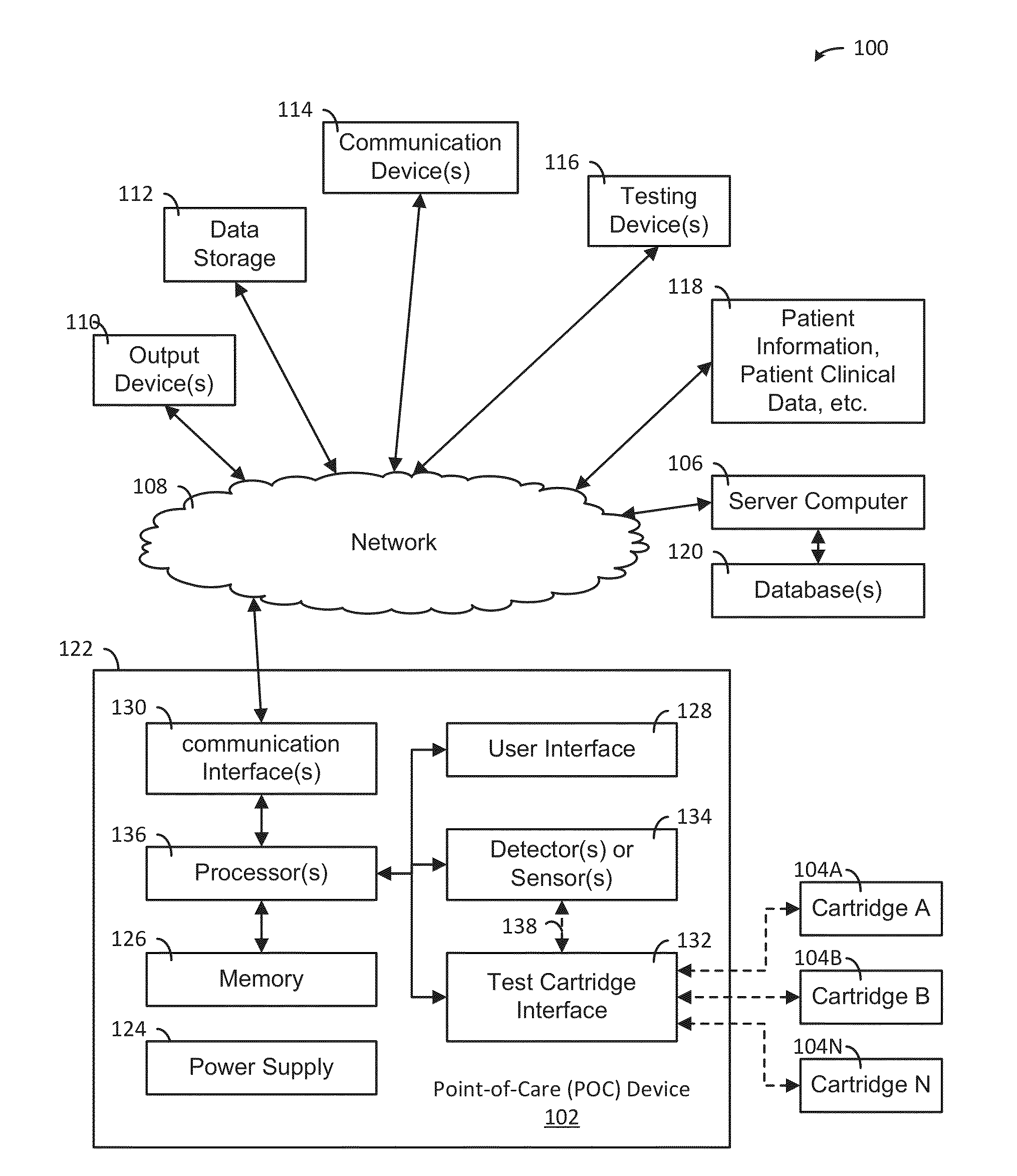
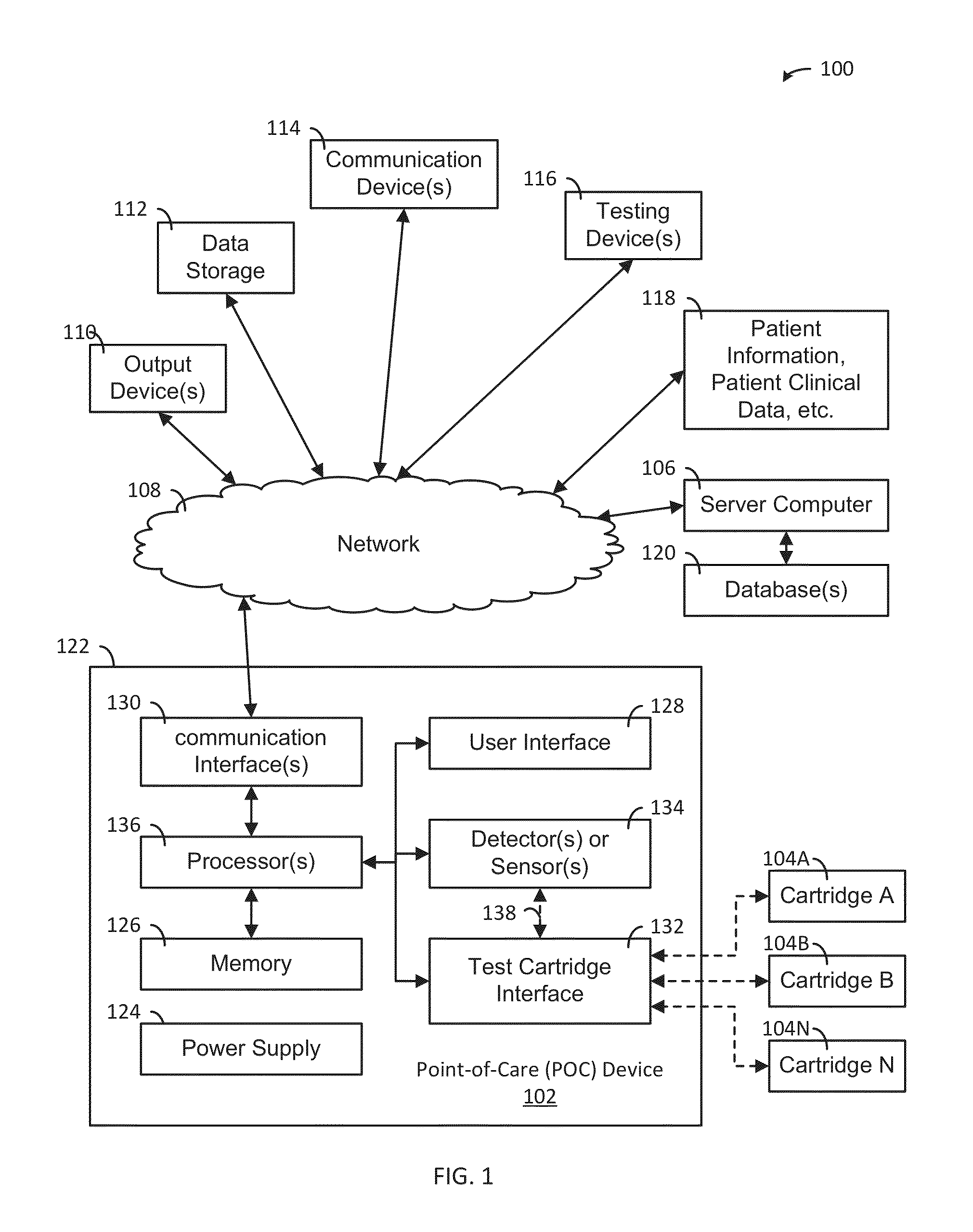
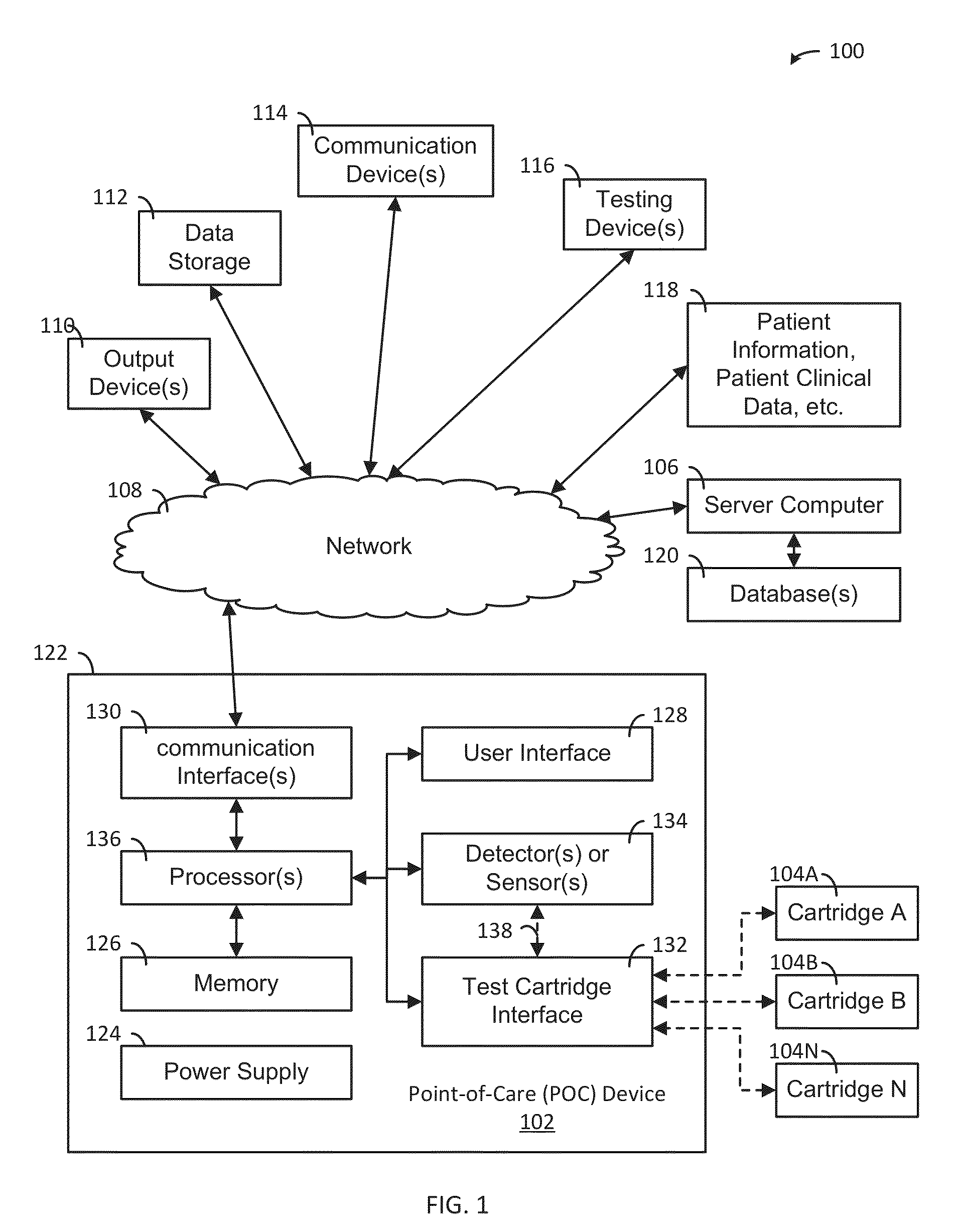
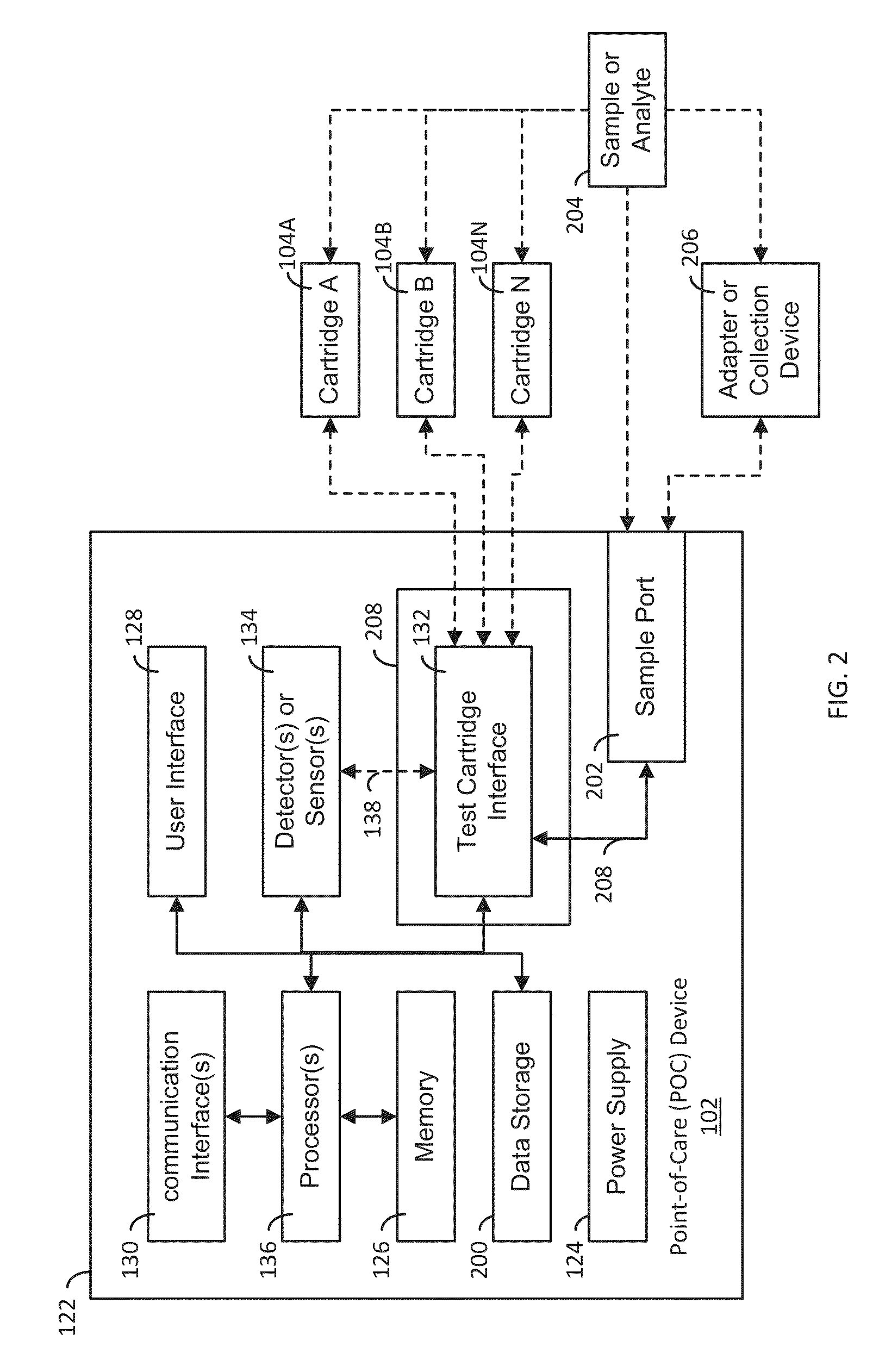
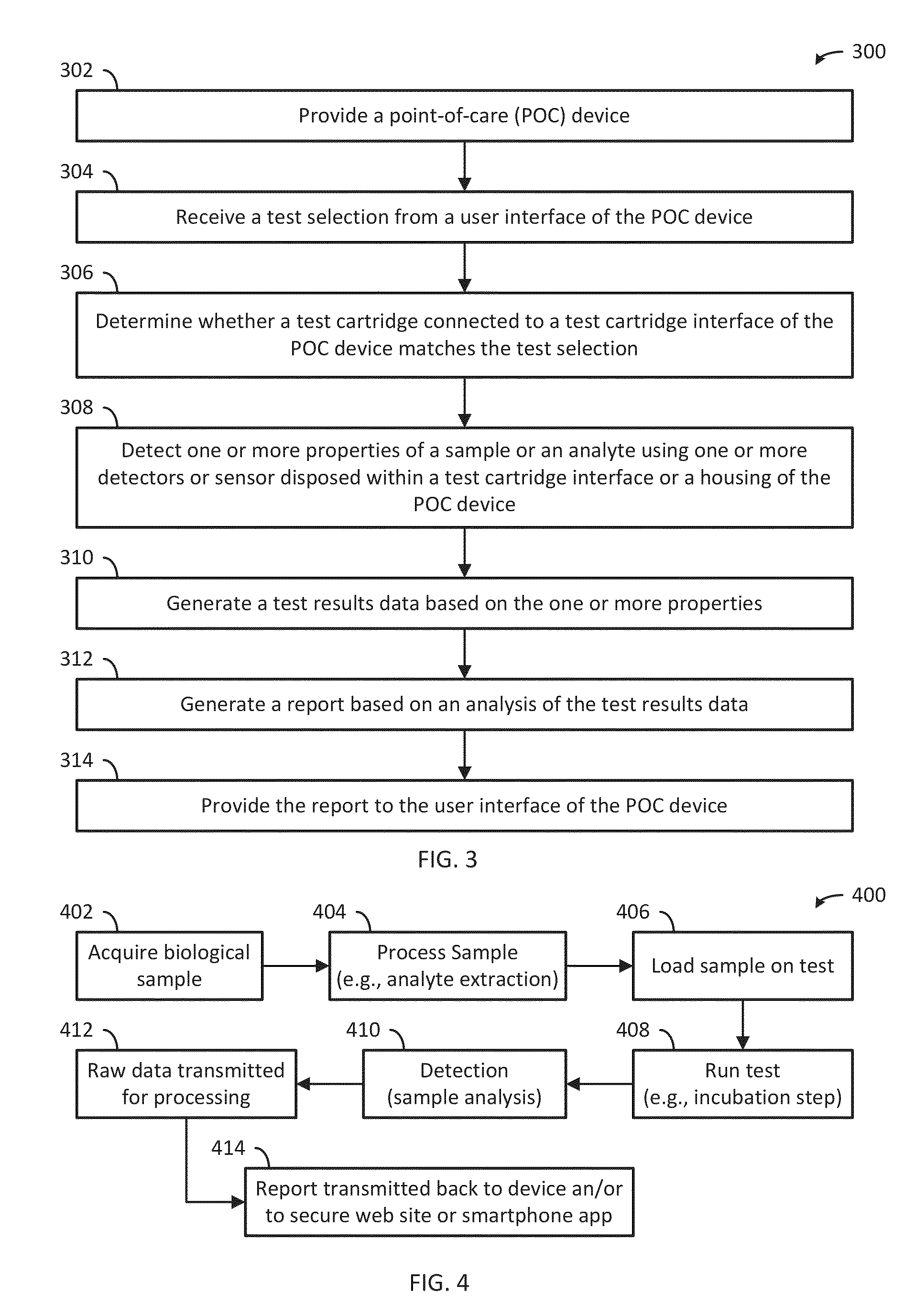
A system, apparatus and method evaluates samples or analytes using a point-of-care device. A test selection is received from the user interface. A determination is made whether a test cartridge connected to the test cartridge interface matches the test selection. Properties of the sample or the analyte are detected using detector(s) or sensor(s) in the POC device. A test results data based on the properties is generated. A report based on an analysis of the test results data is generated and the report is provided to the user interface of the POC device. The POC device also includes memory, communication interface(s), test cartridge interface, and processor(s).
Owner:PANDORA GENOMICS
Micro paddle wheel pump for precise pumping, mixing, dispensing, and valving of blood and reagents
InactiveUS20030072647A1Rotary stirring mixersTransportation and packagingImpellerPoint of care device
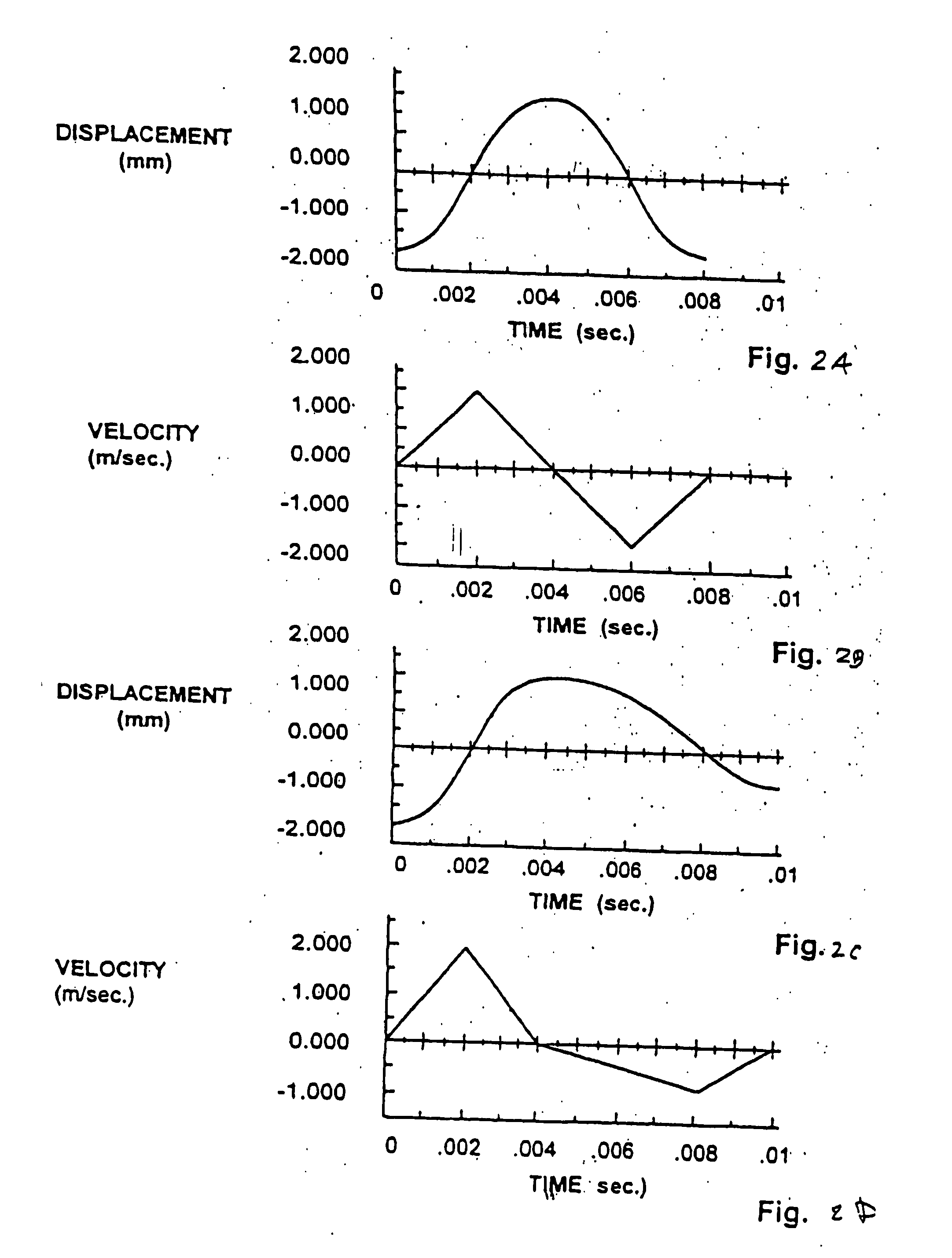
An apparatus and method for making a microscopic paddle wheel coupled inductively by an external electromagnet and used for valving and active pumping so that the actual pumping mechanism is completely isolated from the electromagnetic driver. The paddle wheel is inexpensive to manufacture and disposable. A cartridge having a network of conduits and reservoirs contains several of such paddle wheels to transport blood and reagents. A point-of-care device houses the electromagnetic driving mechanism and is reused with successive cartridges since the paddle wheels are contained by the cartridge and do not contaminate the driving mechanism.
Owner:SANOFI AVENTIS DEUT GMBH
Method and Apparatus for Ultrasonic Determination of Hematocrit and Hemoglobin Concentrations
ActiveUS20070266778A1Simple accurate quick measurementVibration measurement in solidsUltrasonic/sonic/infrasonic diagnosticsMedicinePoint of care device
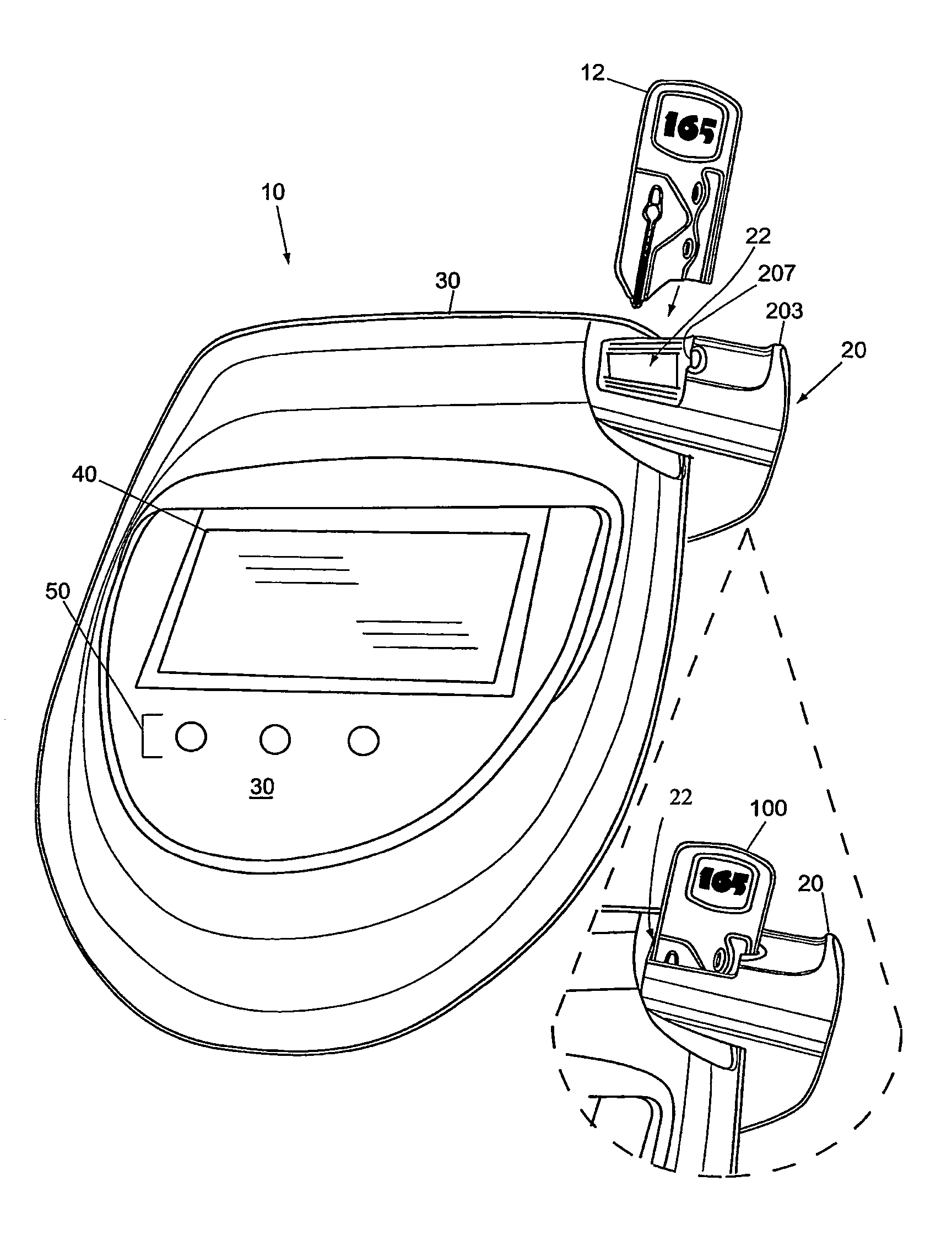
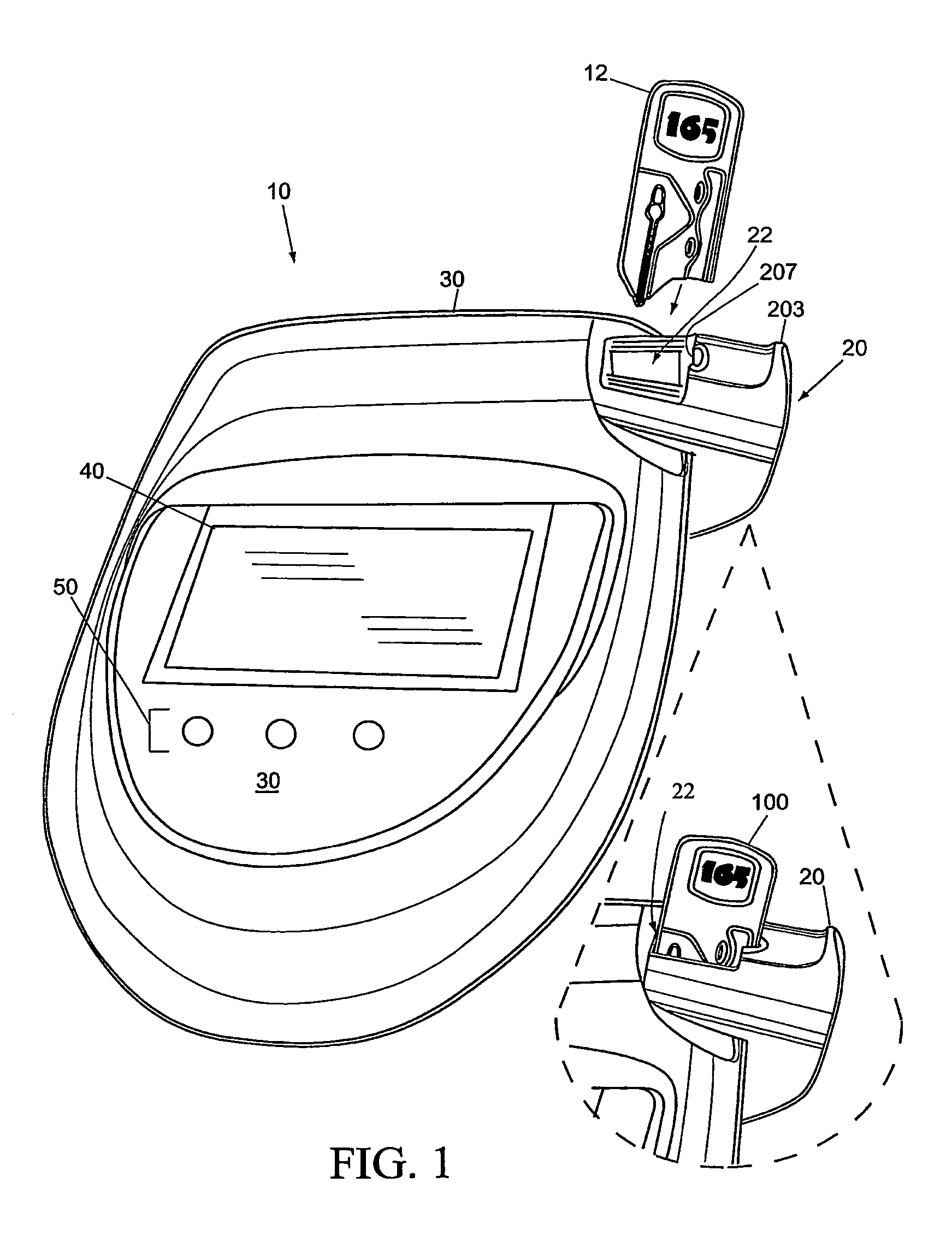
An ultrasonic field-portable system for accurately measuring hematocrit (HCT) and hemoglobin concentration (HGB) in small food samples. The system includes an analyzer (10) that allows extremely accurate measurements of blood hematocrit from only one or two drops of +>>d collected in a disposable sampling device (12) that is then inserted into the analyzer (10). The system is compact enough to package into a point of care device, making it a point of care device with accuracy comparable to larger CBC lab equipment.
Owner:SEPARATIONS TECH
Methods and Devices for Determining Sensing Device Usability
ActiveUS20130002278A1Material analysis by electric/magnetic meansElectrical testingElectricityPoint of care device
Methods and devices for determining sensing device usability, e.g., for self-monitoring and point of care devices. In one embodiment, the invention is to a method of determining device usability, comprising the steps of providing a device comprising a first electrical pad; a second electrical pad; and a humidity-responsive polymer layer contacting at least a portion of the first and second electrical pads; applying a potential across the first and second electrical pads; measuring an electrical property associated with the humidity-responsive polymer layer; and determining whether the measured electrical property associated with the humidity-responsive polymer layer has exceeded a humidity threshold level associated with the device usability.
Owner:ABBOTT POINT CARE
Method and apparatus for ultrasonic determination of hematocrit and hemoglobin concentrations
ActiveUS7523649B2Simple accurate quick measurementUltrasonic/sonic/infrasonic diagnosticsVibration measurement in solidsPoint of care deviceBiomedical engineering
An ultrasonic field-portable system for accurately measuring hematocrit (HCT) and hemoglobin concentration (HGB) in small food samples. The system includes an analyzer (10) that allows extremely accurate measurements of blood hematocrit from only one or two drops of +>>d collected in a disposable sampling device (12) that is then inserted into the analyzer (10). The system is compact enough to package into a point of care device, making it a point of care device with accuracy comparable to larger CBC lab equipment.
Owner:SEPARATIONS TECH
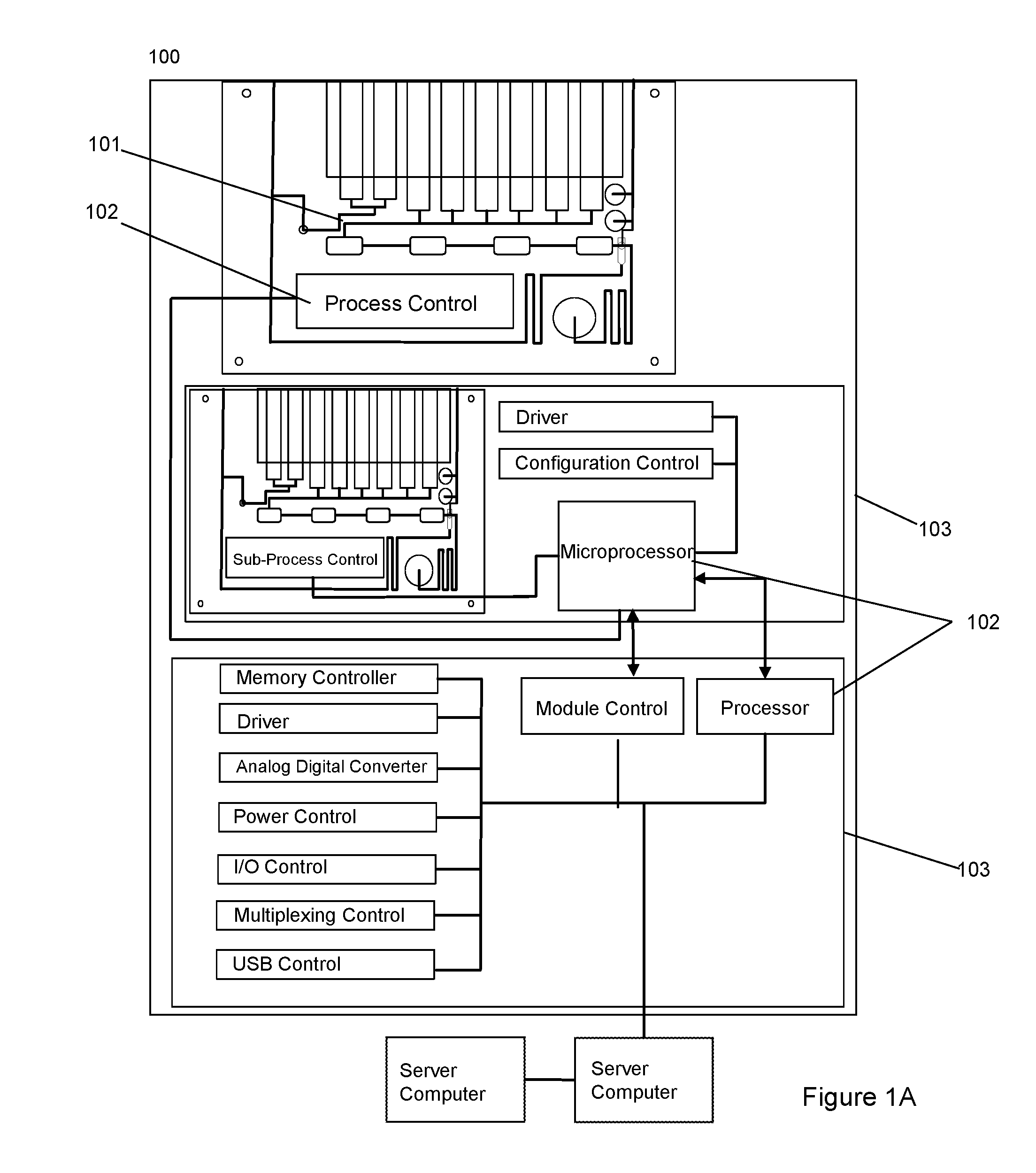
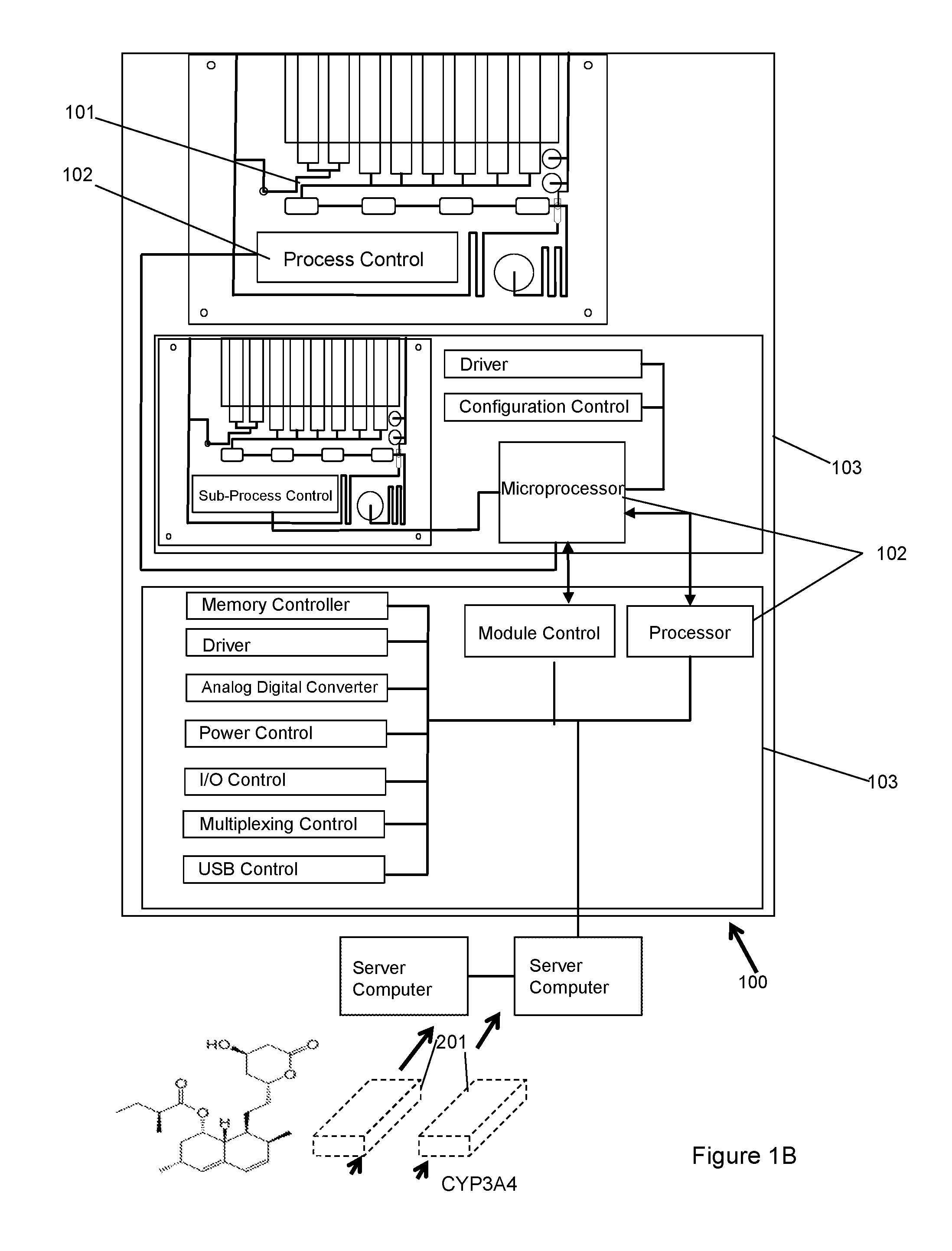
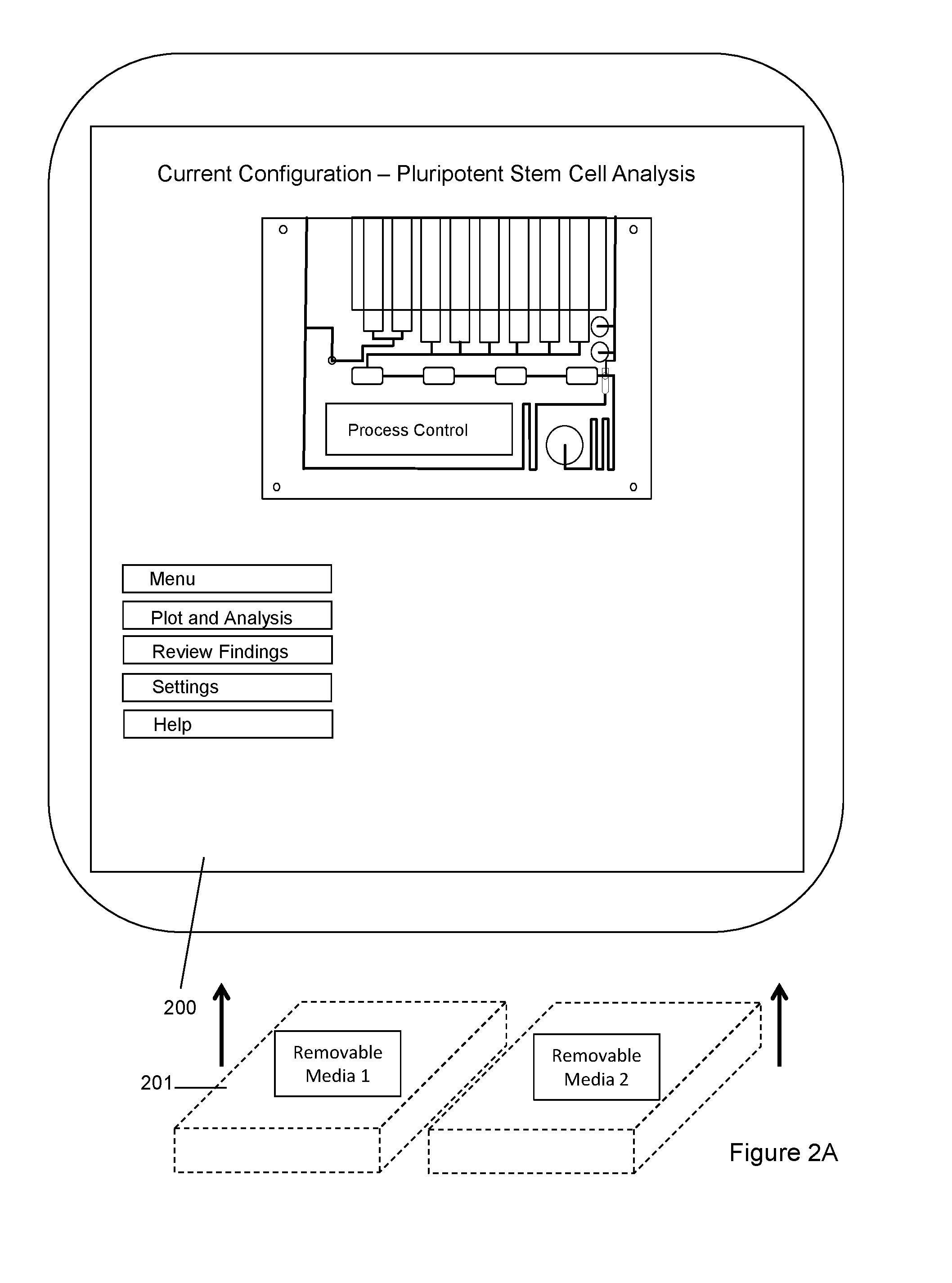
Dynamic Lab on a Chip Based Point-Of-Care Device For Analysis of Pluripotent Stem Cells, Tumor Cells, Drug Metabolites, Immunological Response, Glucose Monitoring, Hospital Based Infectious Diseases, and Drone Delivery Point-of-Care Systems
The invention provides for a novel dynamically configurable point-of-care device for clinical diagnostics and research for analysis of activity associated with pluripotent stem cells, tumor cells, drug metabolites, immunological response, glucose monitoring, cardiovascular diseases, liver cell therapy, cell-cell signaling, epidemic outbreaks, hospital based infectious diseases, pathogens, germ cells, pharmacological compounds, oxidation reduction, microscopy, tomography, flow cytometry, clinical lab testing, and for providing immunoassays, ELISA, electrophoresis, PCR, chromatography, and other laboratory functions. The device comprises a biochemical processing module further comprising a processor and at least one controller, receiving microfluidic elements, sensors, software scripts, an electrically operated interface, flow ports, a user interface, memory, and a communications link, configurable based on analysis of patient data. The invention further provides for multiple-criteria decision analysis for hospital administrators, a wearable device, mobile medical device, molecular electronics configuration, touchscreen recognition, data analytics application, and a drone delivery based point-of-care system.
Owner:PATEL NILESH
Nanostructured electrochemical biosensor with aptamer as molecular recognition probe
InactiveUS20080156646A1Quick fixQuick checkImmobilised enzymesBioreactor/fermenter combinationsElectrochemical biosensorNanostructure
The present invention details a nanostructured electrochemical biosensor based on aptamer as the molecular recognition probe. The biosensor is comprised of an electrochemical cell that can be plugged into an electric controller. This electrochemical biosensor contains a working electrode made of the micro- / nano-scale gold dot array pattern, on which aptamer is immobilized on the surface of the dot array as the molecular recognition probe. The aptamer is labeled with an electrochemical indicator. Reversible binding of an analyte to the aptamer causes the change in the conformation of aptamer, consequently brings the electrochemical indicator close to the electrode surface. This results in the electron transfer from the electrochemical indicator to the electrode, which can be read as a change in output current or potential. The invented biosensor is a portable point-of-care device that has higher sensitivity, allows the measurement of the analyte more rapidly and requires much less sample volume than is presently available with current methods of detection.
Owner:WU NIANQIANG +3
Point of care polymerase chain reaction device for disease detection
InactiveUS20170173585A1Heating or cooling apparatusMicrobiological testing/measurementPoint of care deviceNucleic acid sequencing
A point-of-care device for detecting a target nucleic acid is provided. The device comprises: an extraction chamber adapted to receive a biological sample, wherein said extraction chamber comprises means to extract and lyse the sample to release nucleic acid; a first amplification chamber in communication with the extraction chamber, wherein said amplification chamber comprises means to trigger nucleic acid amplification of a target nucleic acid sequence to occur; and a detection chamber in communication with the amplification chamber, wherein said detection chamber comprises means to detectably label the target nucleic acid and means to detect a signal associated with labeled target nucleic acid.
Owner:ADVANCED THERANOSTICS
Aptamer-Based Device For Detection Of Cancer Markers And Methods Of Use
InactiveUS20120088232A1Improve performanceSugar derivativesComponent separationEnergy transferResonance
Systems and methods are provided for detecting and quantitating one or more compounds or molecules in a sample. An aptamer-based point-of-care device (such as a strip) is described for rapid detection of target molecules such as the cancer marker p-glycoprotein (Pgp). Fluorescent molecules or gold nanoparticles may be used to detect the binding between a target molecule and the aptamer. By way of example, fluorescence resonance energy transfer (FRET) or Dynamic Light Scattering (DLS) may be used for detecting the physical and / or chemical changes caused by the binding of the aptamers to the target molecules.
Owner:MISSOURI STATE UNIVERSITY
Fully integrated hand-held device to detect specific nucleic acid sequences
ActiveUS20190040451A1Enable detectionBiological substance pretreatmentsHeating or cooling apparatusHand heldPoint of care device
A fully integrated and disposable point-of-care device for detecting a target nucleic acid is provided. The device comprises: an extraction chamber adapted to receive a biological sample, wherein said extraction chamber comprises means to extract and lyse the sample to release nucleic acid; a first amplification chamber in communication with the extraction chamber, wherein said amplification chamber comprises means to trigger nucleic acid amplification of a target nucleic acid sequence to occur; and a detection chamber in communication with the amplification chamber, wherein said detection chamber comprises means to detectably label the target nucleic acid and means to detect a signal associated with labeled target nucleic acid, or a single chamber for amplification, detection and identification of multiple nucleic acid sequences.
Owner:ADVANCED THERANOSTICS
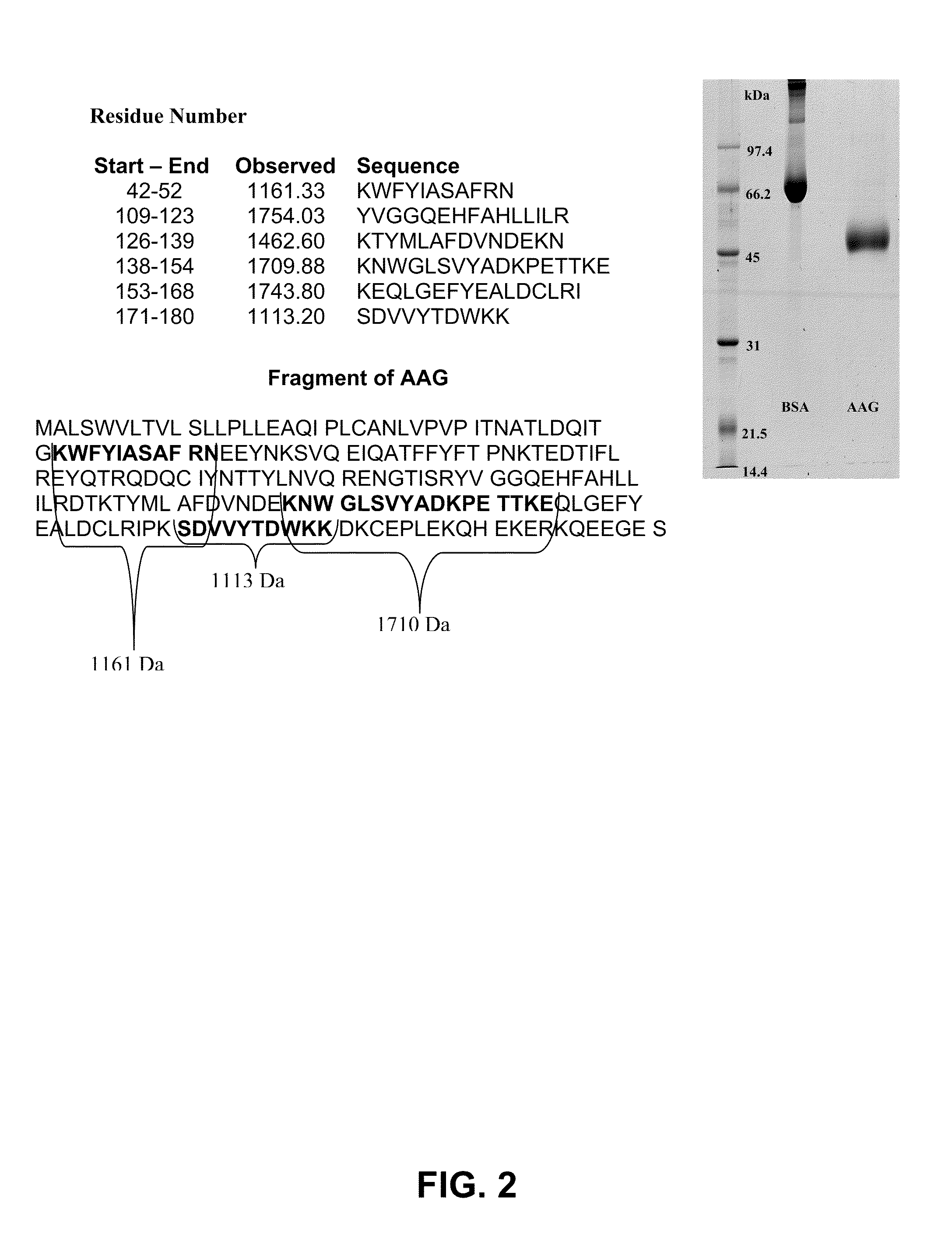
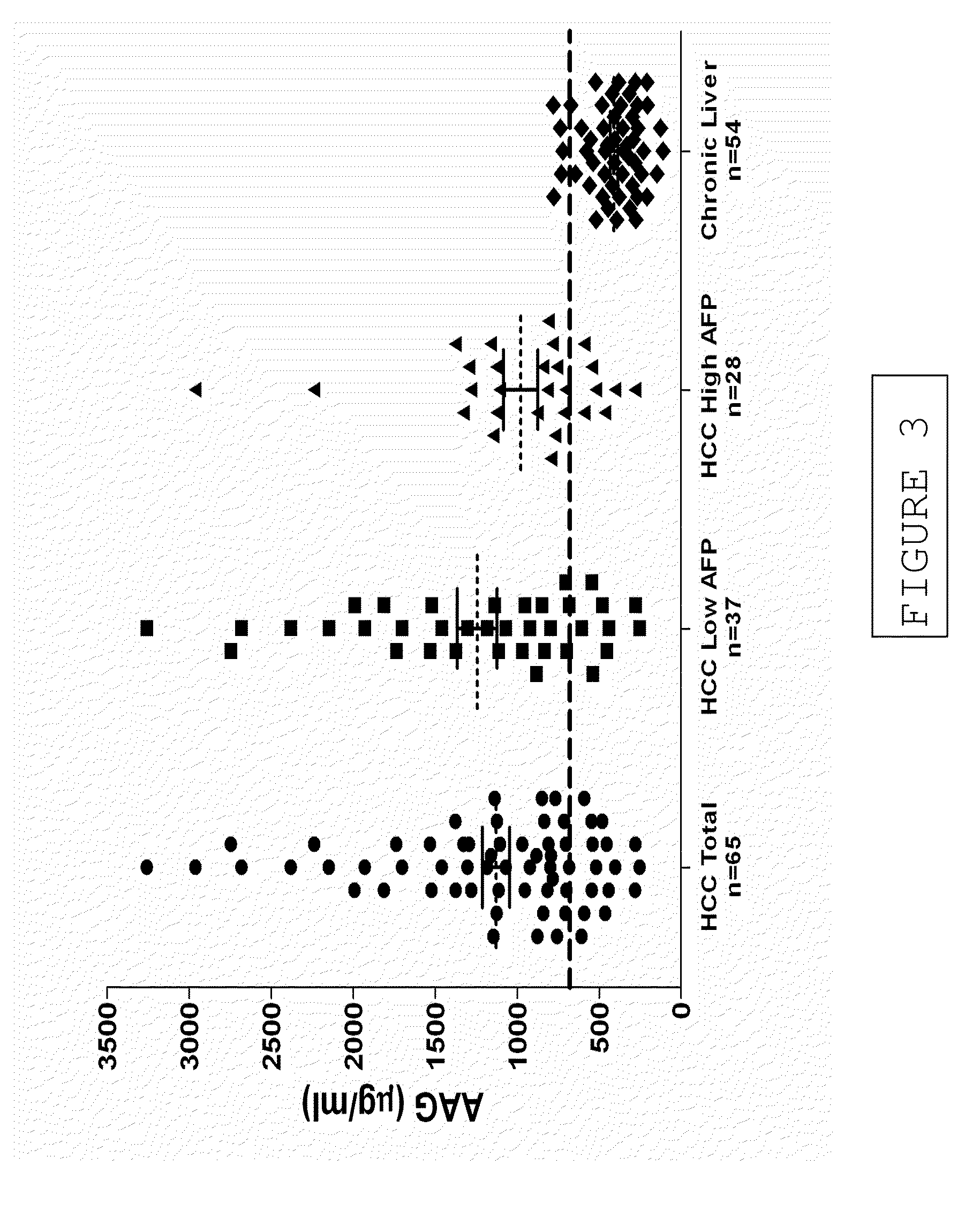
Diagnostic test for hepatocellular carcinoma
InactiveUS20090317844A1High sensitivityImprove accuracyBioreactor/fermenter combinationsBiological substance pretreatmentsDiagnostic testTissue sample
A diagnostic test for hepatocellular carcinoma is based on increases in the concentration of two biochemical markers in a biological sample, alpha-1-acid glycoprotein (AAG) or isoforms thereof and alpha-fetoprotein (AFP) or glycoforms thereof. Levels of these markers may be performed using an immunoassay. Also disclosed is a diagnostic kit for use in these assays that comprises reagents for detecting and / or measuring in AAG and AFP in blood, serum or tissue samples. A point-of-care device for the performance of such measurements is also disclosed.
Owner:MOCHTAR RIADY INST OF NANOTECH
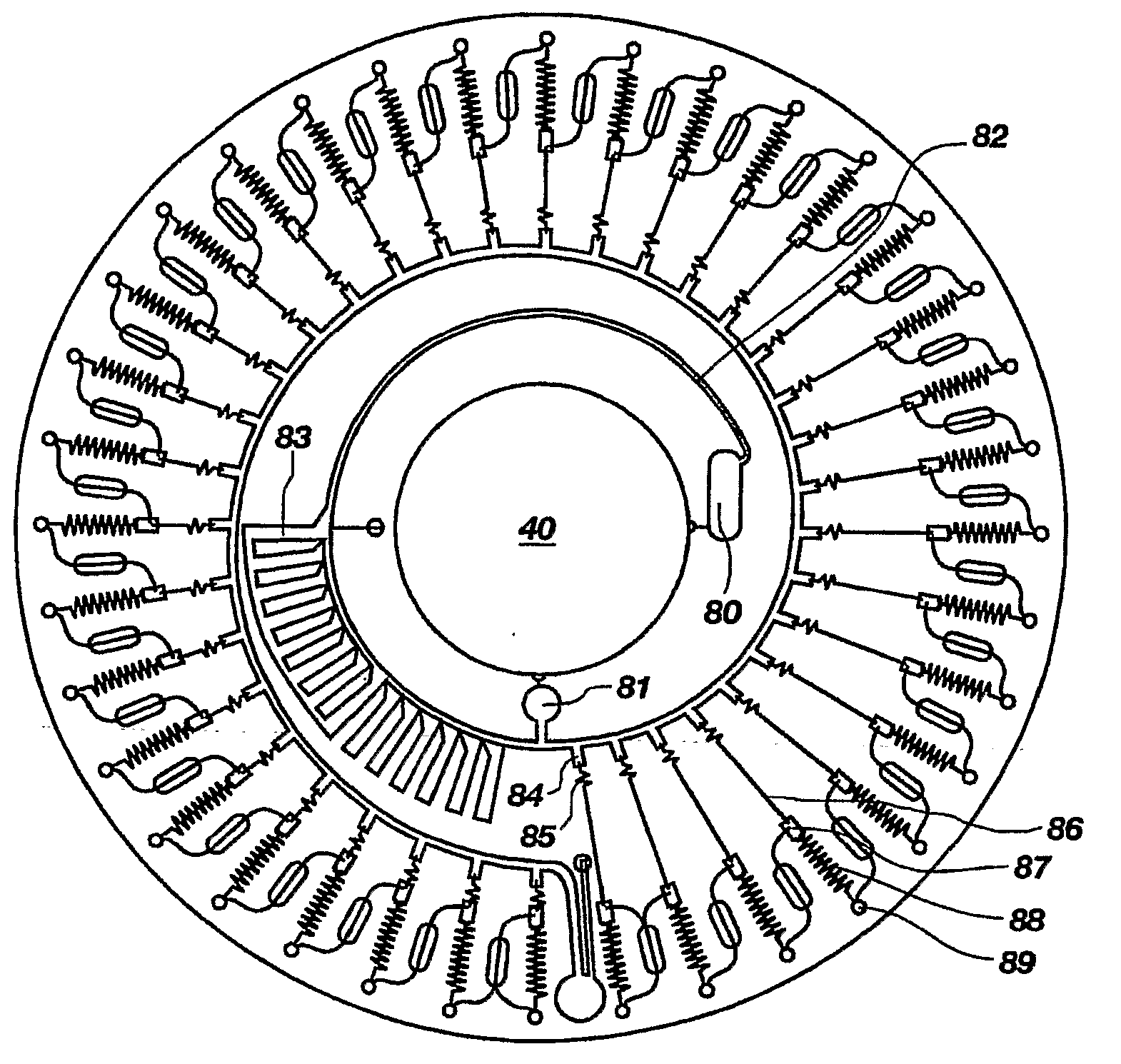
Bioluminescence-based sensor with centrifugal separation and enhanced light collection
InactiveUS20090104643A1Bioreactor/fermenter combinationsBiological substance pretreatmentsPoint of care deviceLuminescence
In general, embodiments of the present invention relate to a bioluminescence-based point of care device that is made up of at least one reaction well (89) that contains a bioluminescent reagent for a luminescent reaction, sample well (80), sample collection well (84), and reagent well (87). A sample is introduced into the reaction wells (89), where it dissolves the reagents and initiates the luminescent reaction, where a luminescence signal is then transmitted through a window to a photo detector.
Owner:UNIV OF UTAH RES FOUND
System, apparatus and method for evaluating samples or analytes using a point-of-care device
ActiveUS9417210B2Easy to integrateEasy to implementMaterial analysis by electric/magnetic meansBiostatisticsCommunication interfaceAnalyte
Owner:PANDORA GENOMICS
Automated work station for point-of-care cell and biological fluid processing
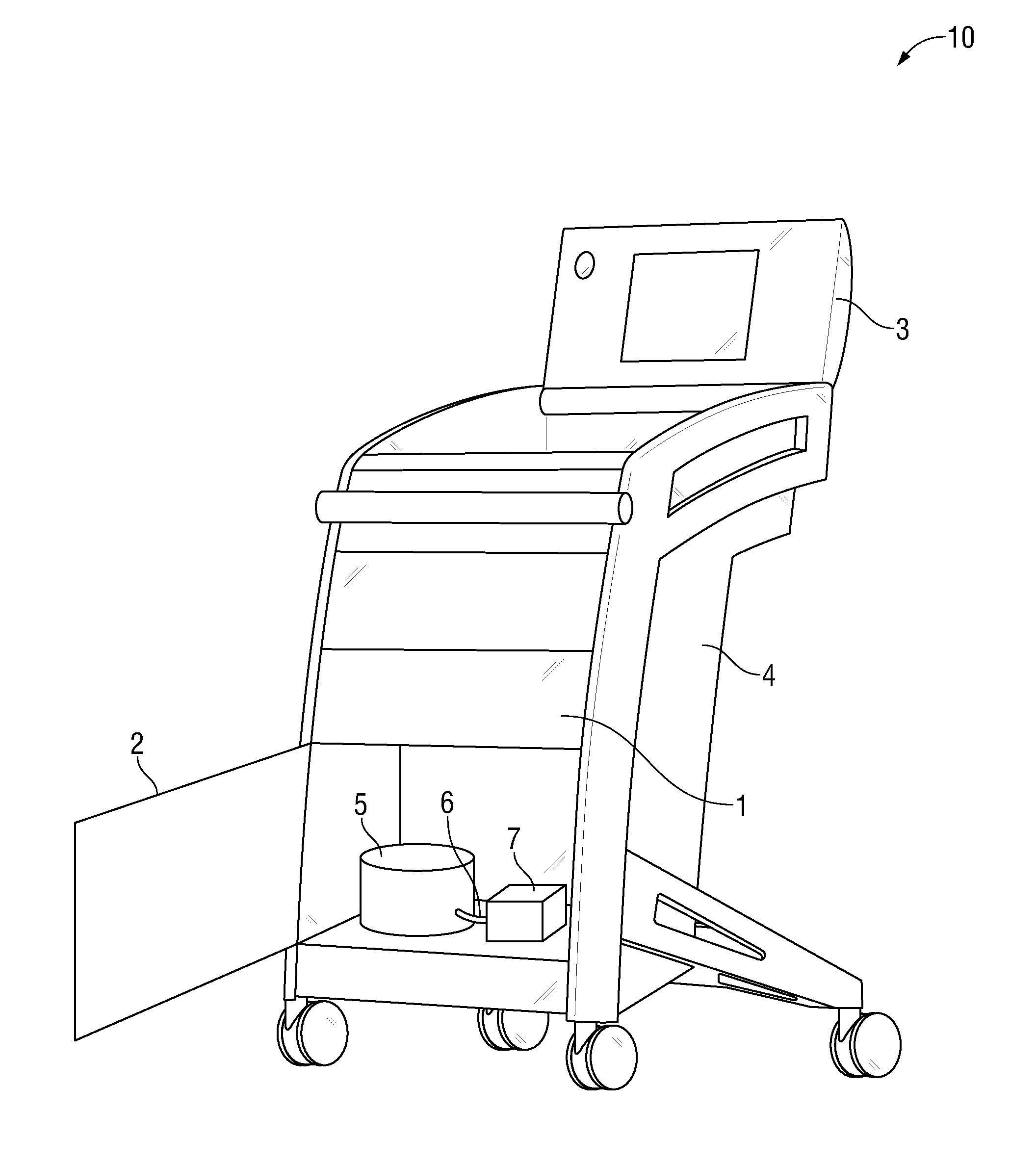
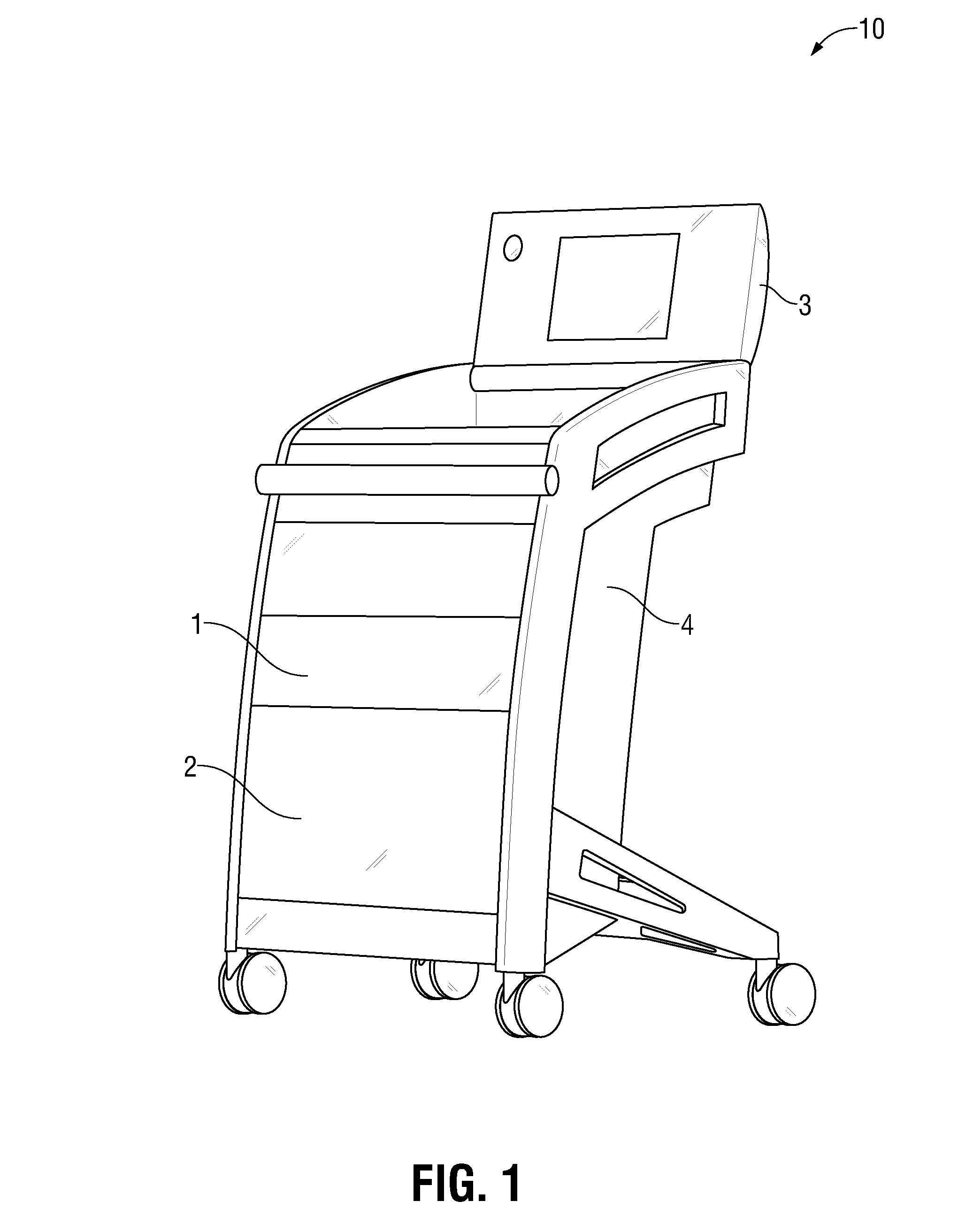
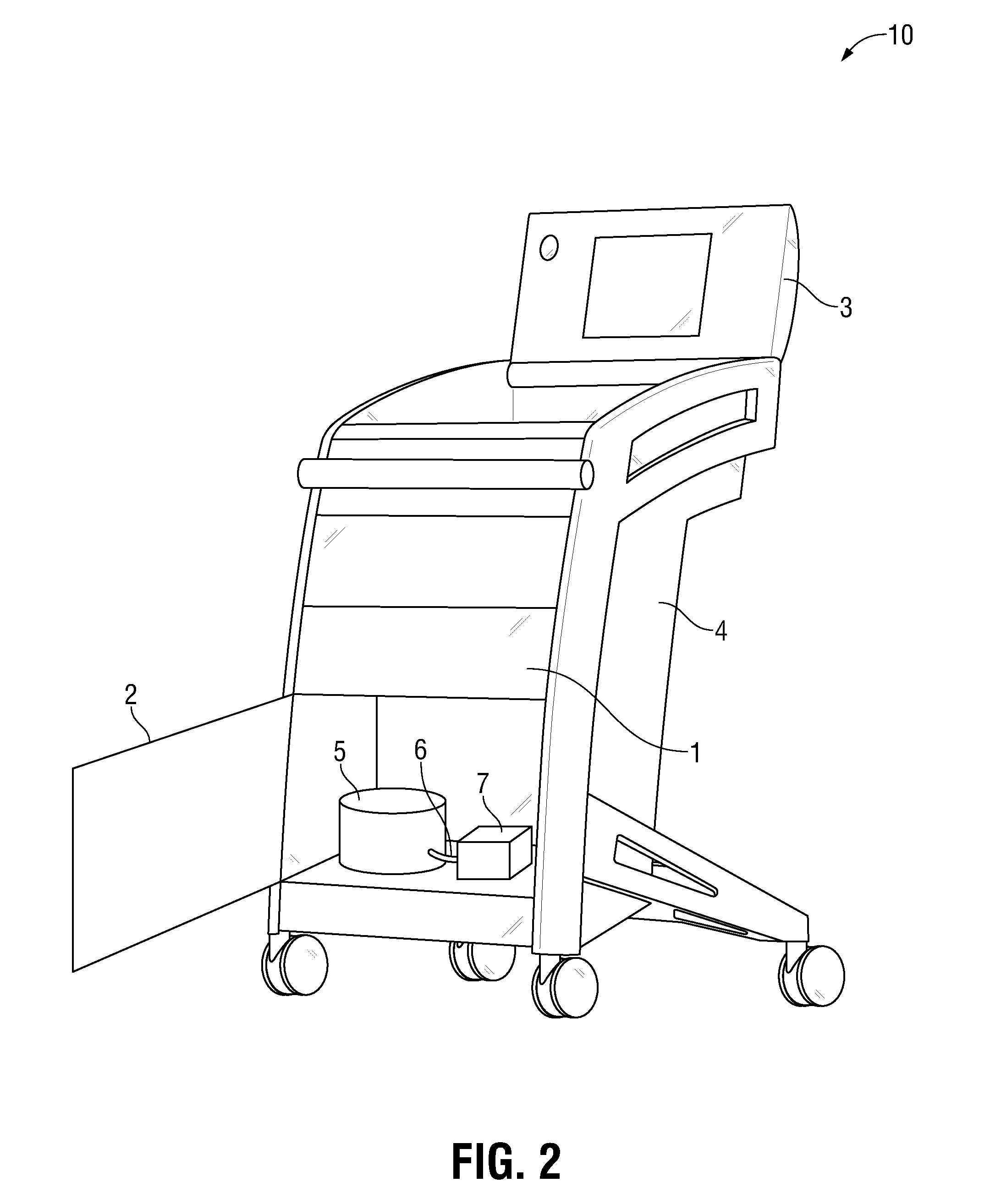
The invention is directed to a work station for use in performing processing of patient samples, storing equipment and drugs in a hospital and the like, the cart having a lower housing having caster wheels mounted on the bottom thereof, the lower housing having angular-shaped side panels therearound which may be opened for entrance into all sides of the housing, and one or more of the side panels; an upper housing mounted on top of the lower housing, the upper housing having one or more side panels therearound; and one or more work areas mounted on top of the upper housing, the shelf adapted for receiving point-of-care equipment.
Owner:SPINESMITH PARTNERS
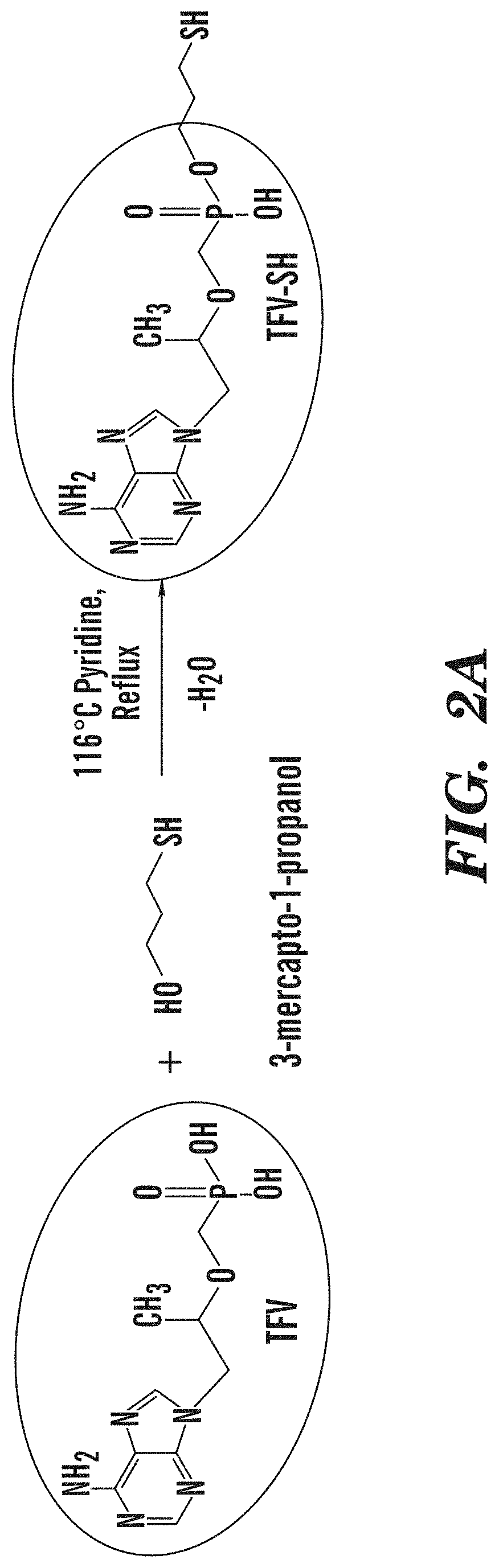
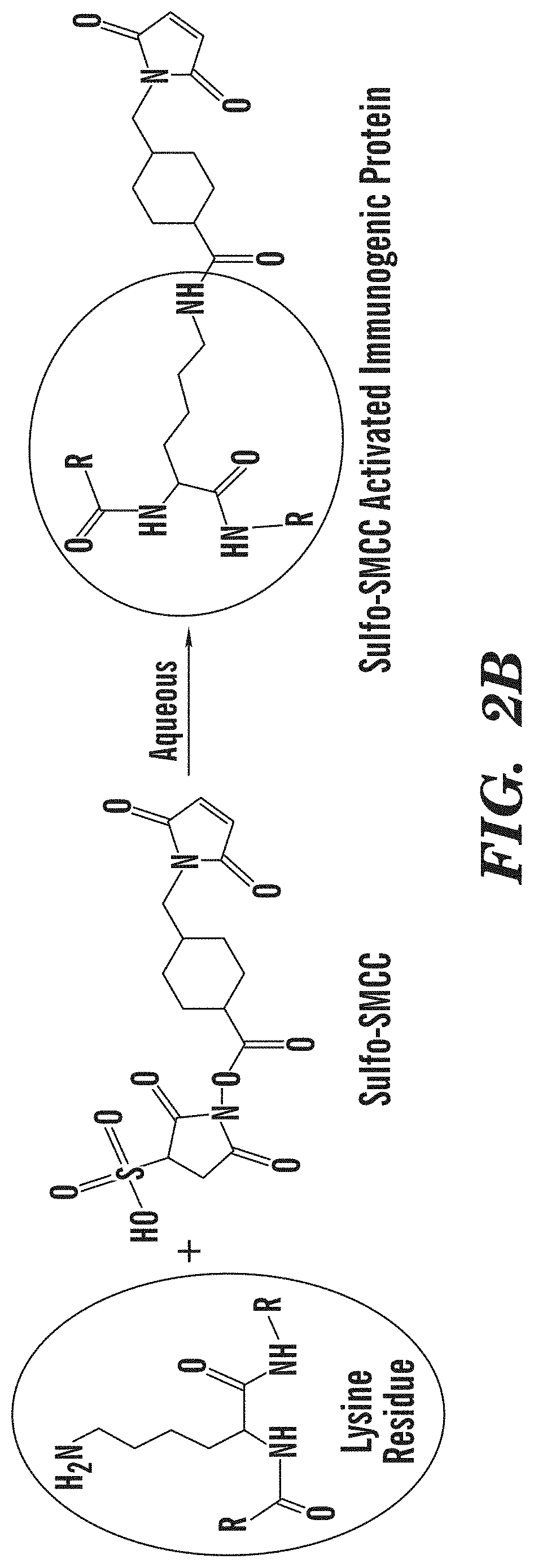
Tenofovir detection assay
ActiveUS10768185B2InhibitionPrevents the virus from multiplyingImmunoglobulinsBiological testingAssayPoint of care device
Owner:TRUSTEES OF BOSTON UNIV
Detection of allergen-specific IgE
InactiveUS20050214866A1Low cost point-of-carePerformed simply and quicklyBioreactor/fermenter combinationsBiological substance pretreatmentsPoint of care deviceImmunotherapy
This invention relates to point-of-care devices and methods to screen animals with suspected IgE-mediated allergic disease. The present invention provides a simple and rapid preliminary immunoassay screen, in which a defined mixture of clinically relevant allergens is used to capture allergen-specific IgE present in a sample, followed by a second binding reagent that selectively binds IgE. The present invention further provides methods for prescribing immunotherapy treatment in animals having IgE-mediated diseases. Devices and kits useful for carrying out the methods of the invention are also provided.
Owner:MCCALL CATHERINE A +2
Methods and Devices for Determining Sensing Device Usability
ActiveUS20160054248A1Resistance/reactance/impedenceMaterial resistanceElectricityPoint of care device
Owner:ABBOTT POINT CARE
Non-Invasive Diagnostic Device for Early Detection of Infection and Inflammation and Methods of Use
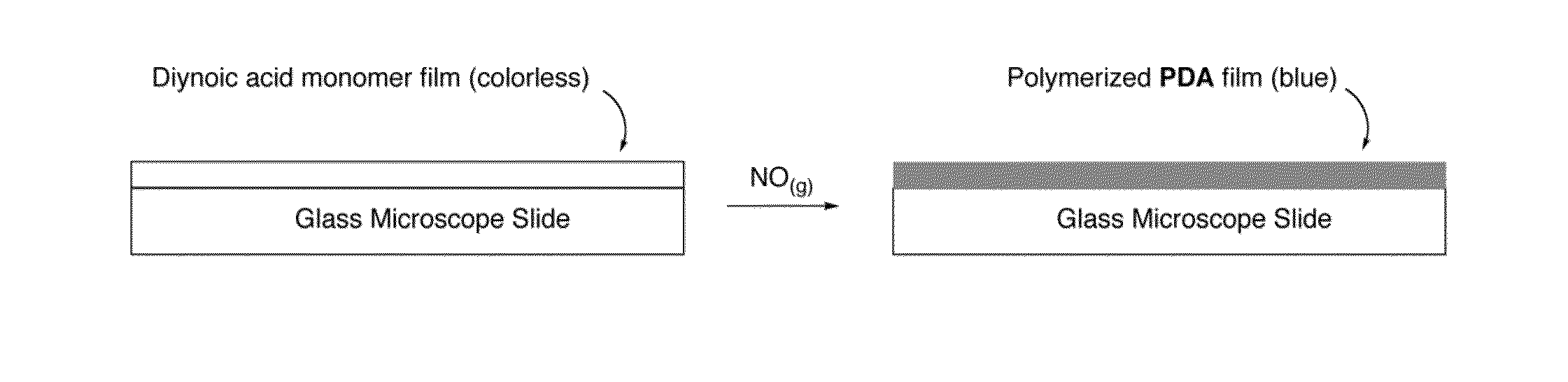
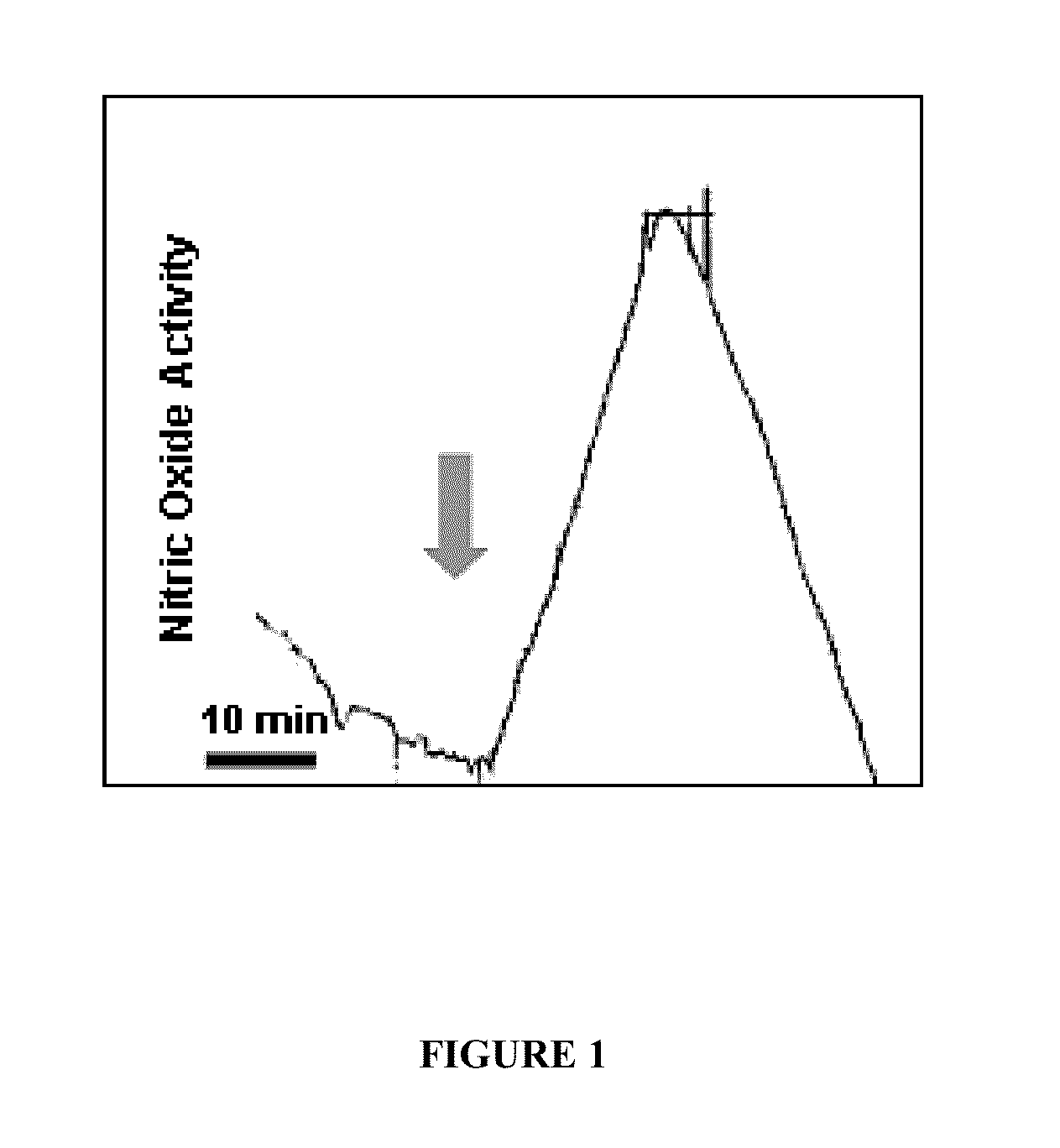
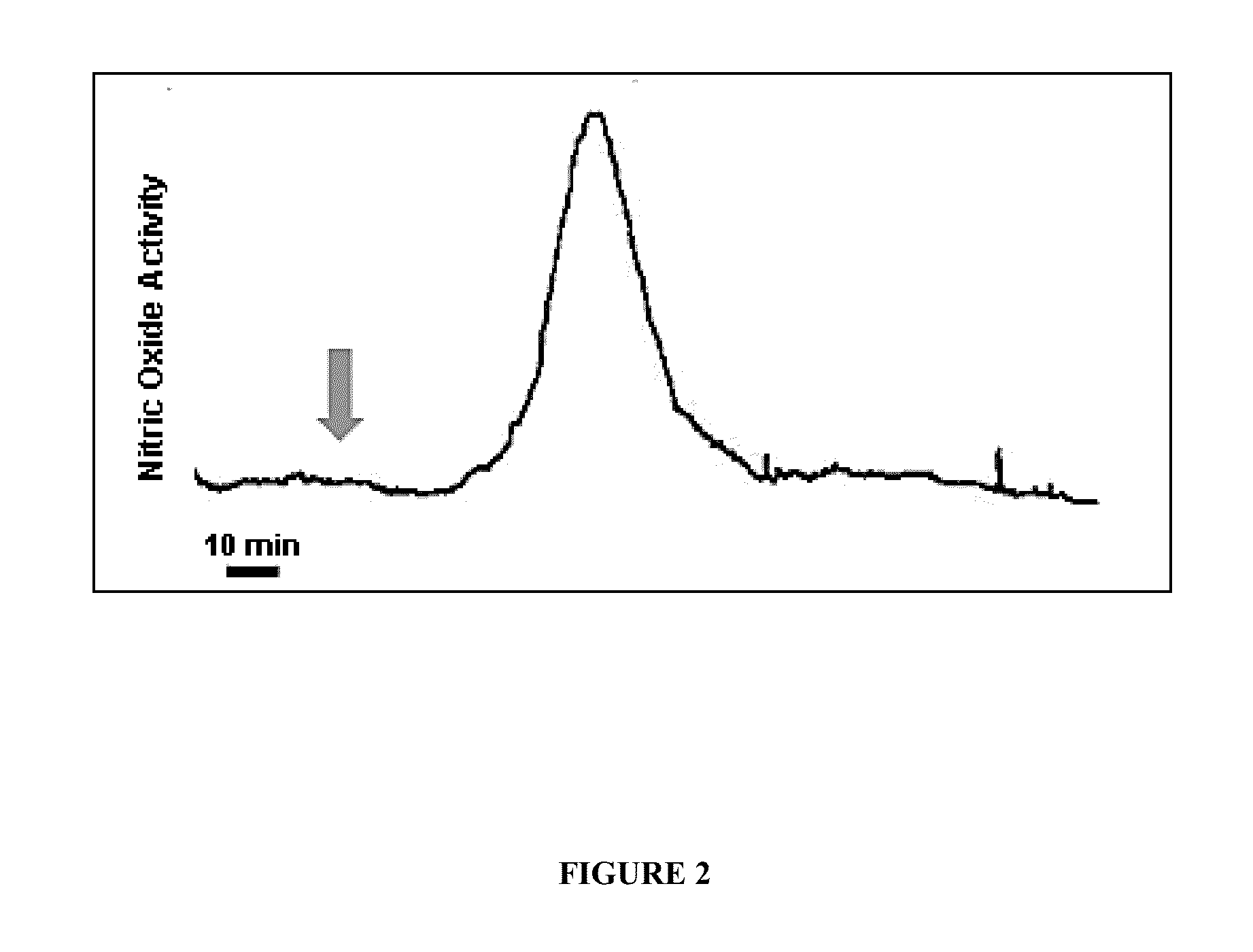
The present disclosure provides, in part, a self-contained, point-of-care device for the early detection of infection, or other pathologic conditions that can lead to system inflammation, by the detection of the endogenous gaseous mediator nitric oxide (NO.) and methods of use.
Owner:DUKE UNIV
Methods and devices for determining sensing device usability
Methods and devices for determining sensing device usability, e.g., for self-monitoring and point of care devices. In one embodiment, the invention is to a method of determining device usability, comprising the steps of providing a device comprising a first electrical pad; a second electrical pad; and a humidity-responsive polymer layer contacting at least a portion of the first and second electrical pads; applying a potential across the first and second electrical pads; measuring an electrical property associated with the humidity-responsive polymer layer; and determining whether the measured electrical property associated with the humidity-responsive polymer layer has exceeded a humidity threshold level associated with the device usability.
Owner:ABBOTT POINT CARE
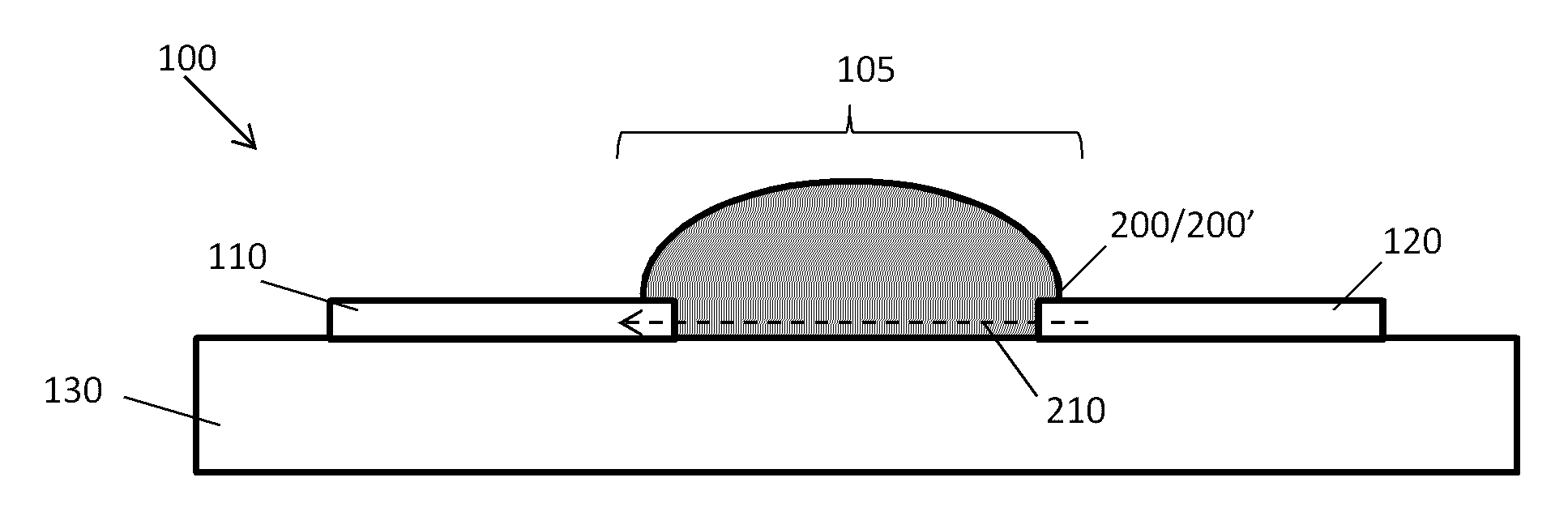
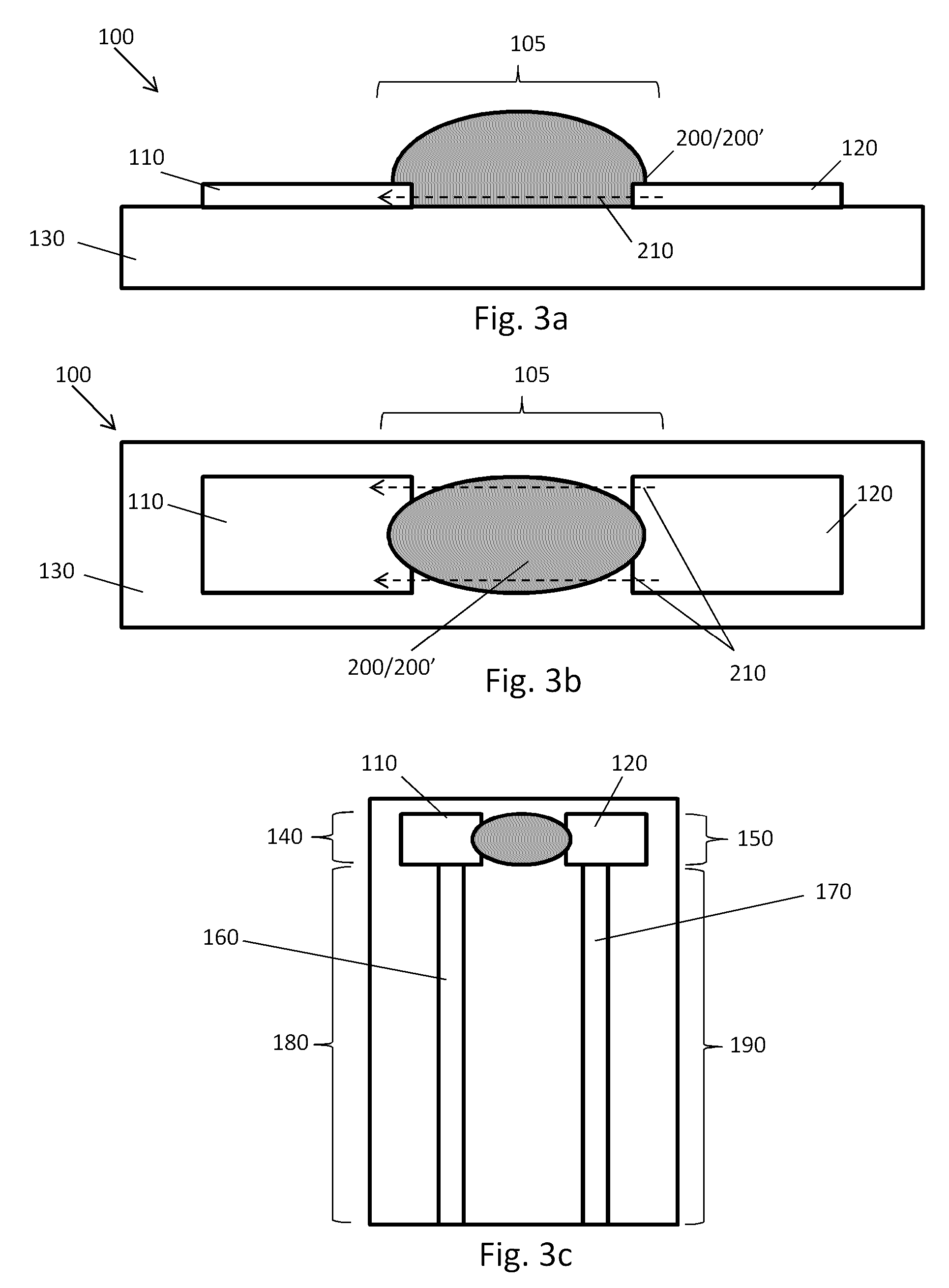
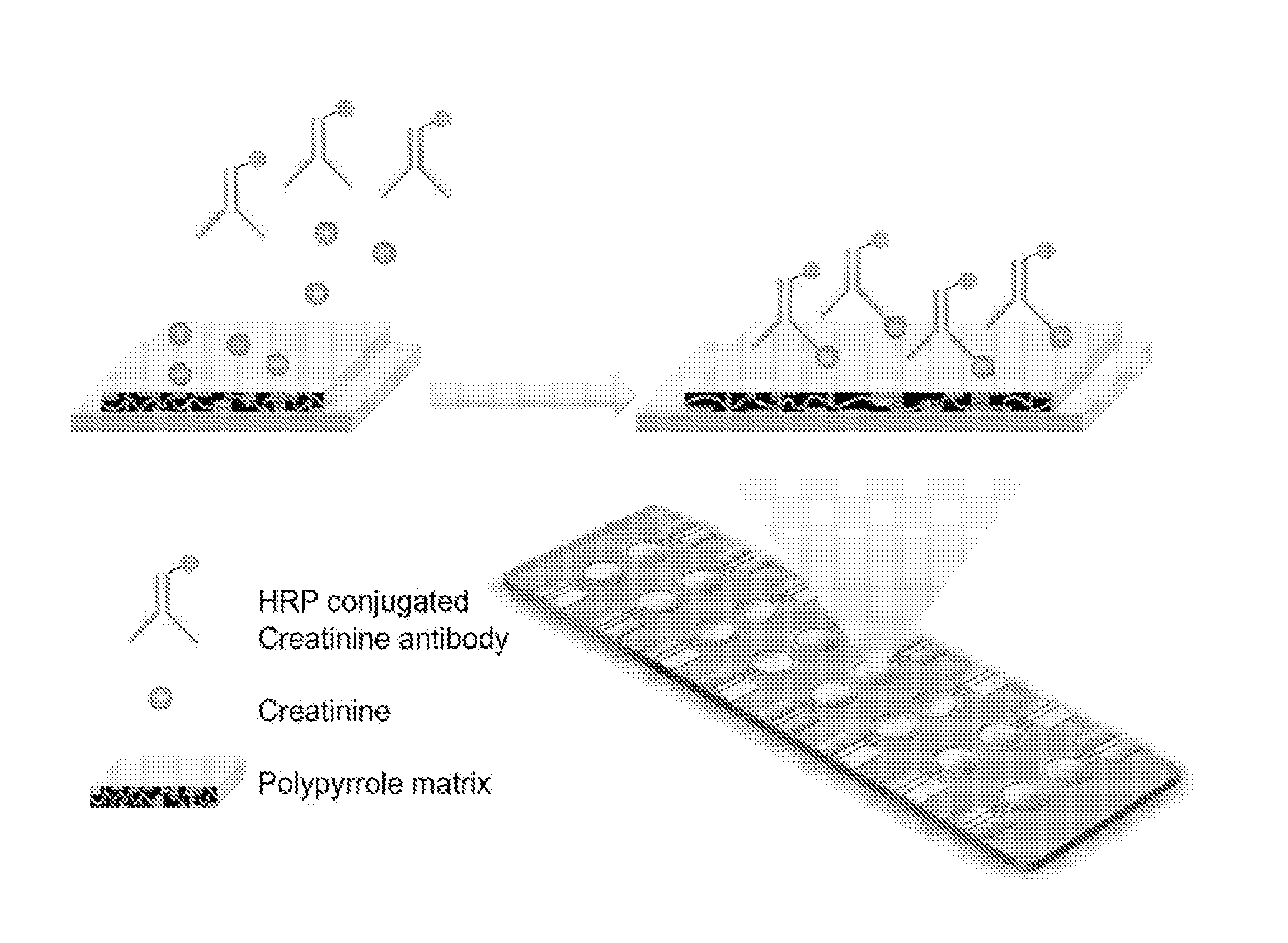
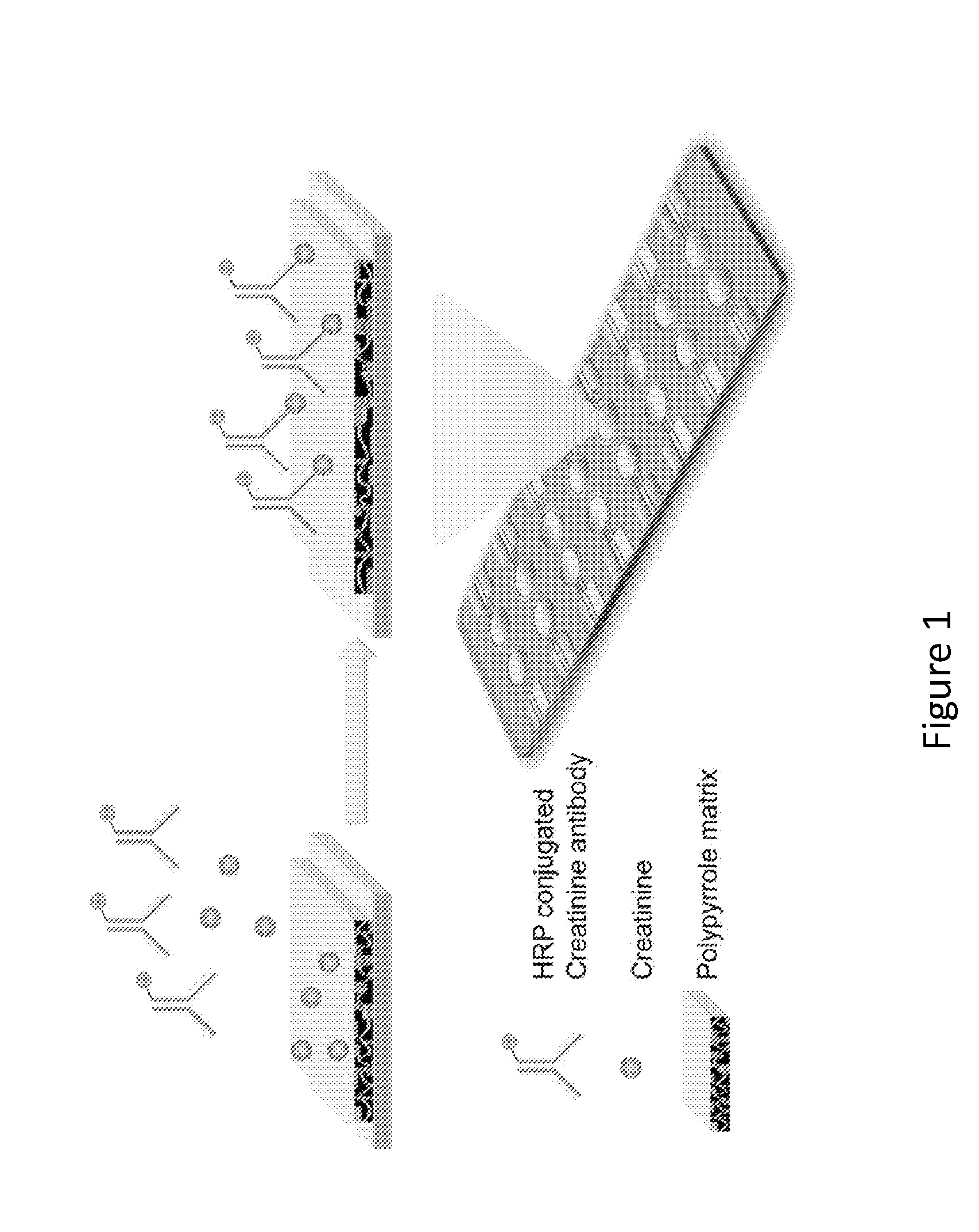
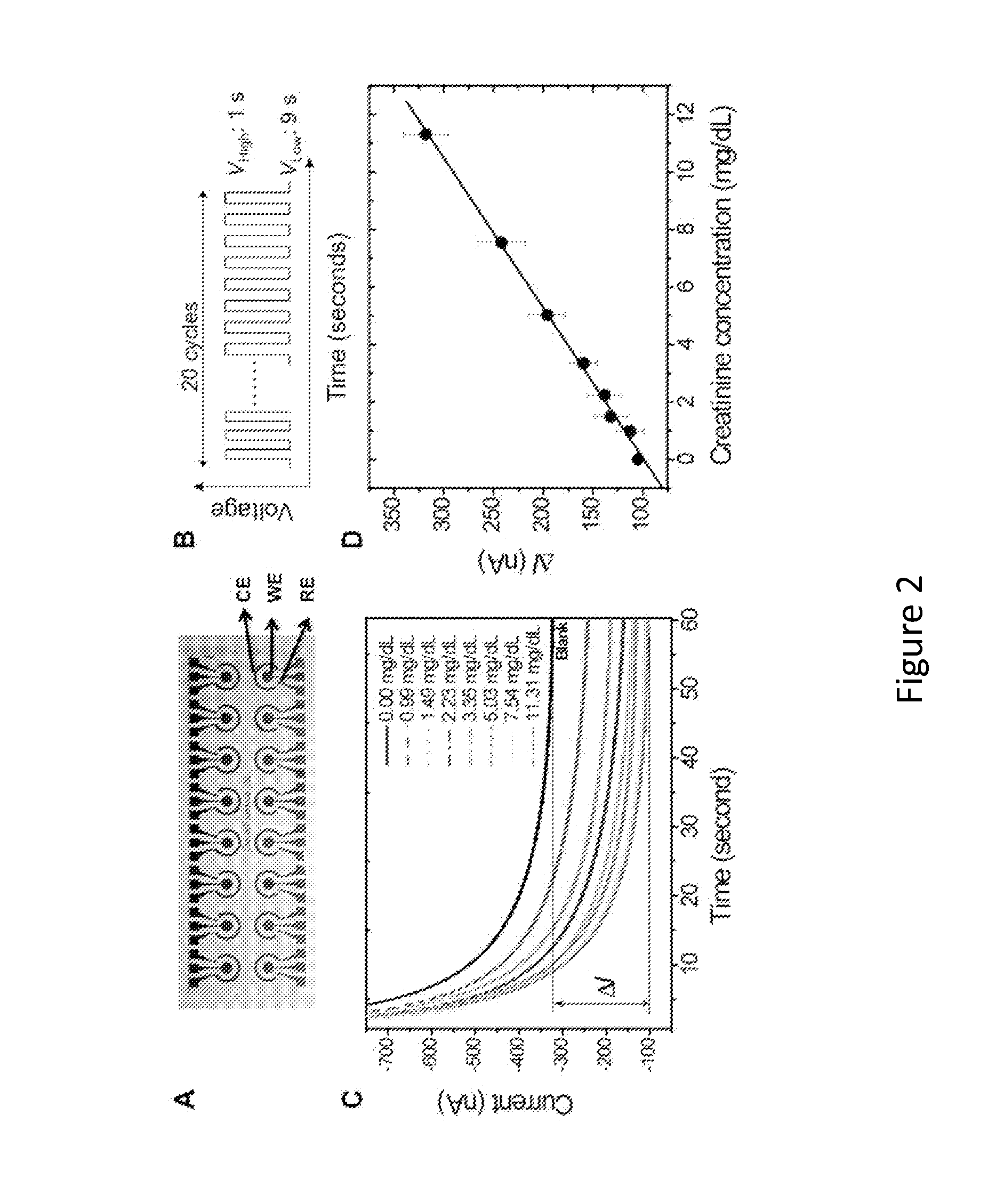
Point-of-Care Device for Monitoring Renal Function
InactiveUS20160007900A1Immobilised enzymesBioreactor/fermenter combinationsPoint of care deviceBiomarker (petroleum)
The present invention relates to a rapid and accurate polymer-based electrochemical point-of-care (POC) single platform assay for a multi-biomarker detection from whole blood to monitor renal function, including the identification of allograft dysfunction.
Owner:RGT UNIV OF CALIFORNIA
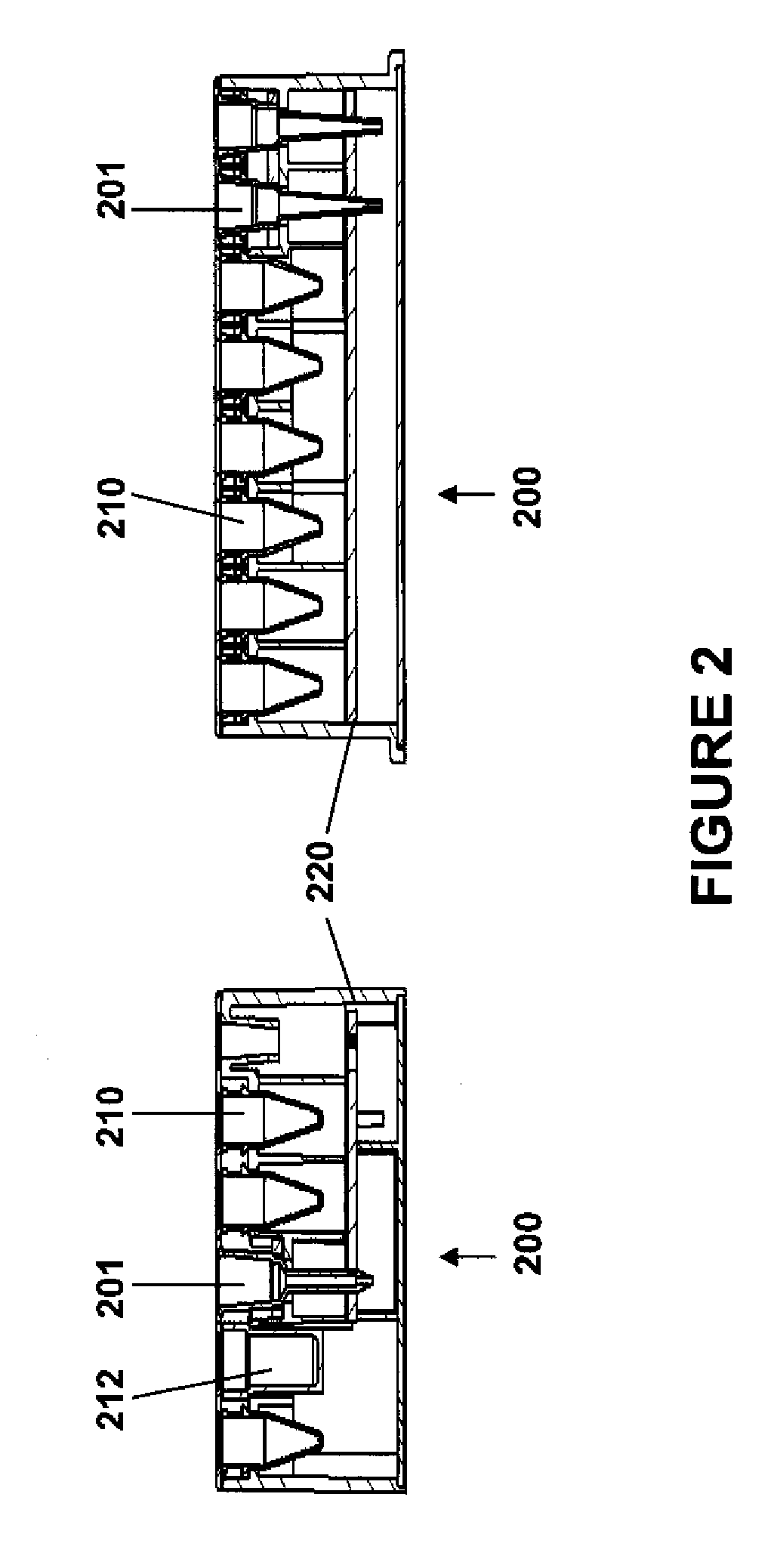
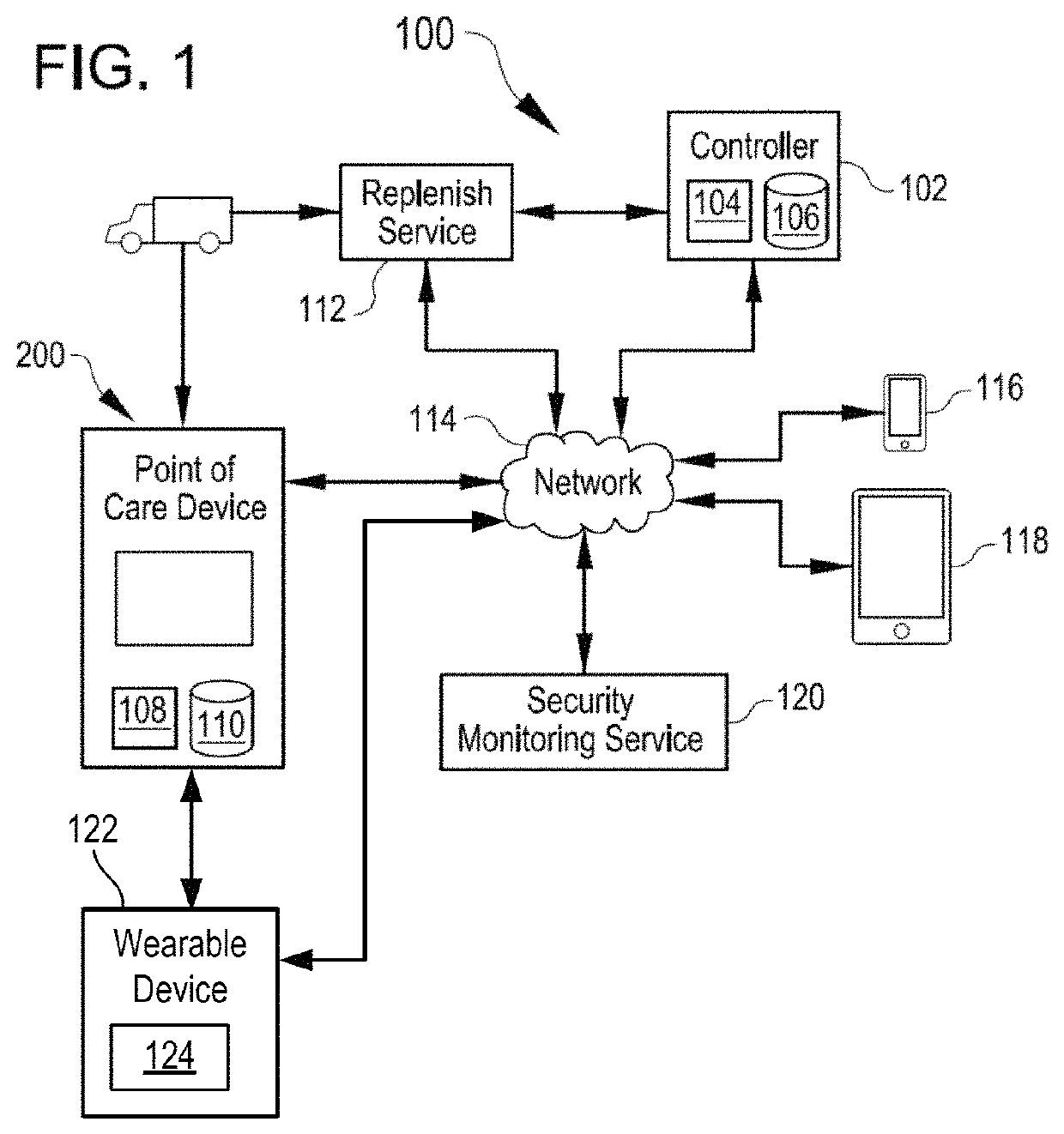
Automated and secure methods for dispensing medication
A point of care device for securely dispensing medication includes an enclosure sized to contain medication containers, a holding element accessible from outside the enclosure to receive medication containers from the enclosure, a dispenser to dispense medication from the enclosure to the holding element, and a securable panel configured to provide access to the enclosure for retrieval or replenishment of the medication containers within the enclosure, and a computing device operable to control the dispenser to dispense medication to the holding element based on various conditions. Specifically, the point of care device can be configured to dispense medication based on detecting that an authorized user is accessing the device, based on a timer in conjunction with known medication regimen, or based on patient or physician demand, or based on biometric information concerning the user.
Owner:TUPELOLIFE SERVICES LLC
Automated work station for point-of-care cell and biological fluid processing
ActiveUS20140110913A1Surgical furnitureDiagnostic recording/measuringPower stationPoint of care device
The invention is directed to a work station for use in performing processing of patient samples, storing equipment and drugs in a hospital and the like, the cart having a lower housing having caster wheels mounted on the bottom thereof, the lower housing having angular-shaped side panels therearound which may be opened for entrance into all sides of the housing, and one or more of the side panels; an upper housing mounted on top of the lower housing, the upper housing having one or more side panels therearound; and one or more work areas mounted on top of the upper housing, the shelf adapted for receiving point-of-care equipment.
Owner:SPINESMITH PARTNERS
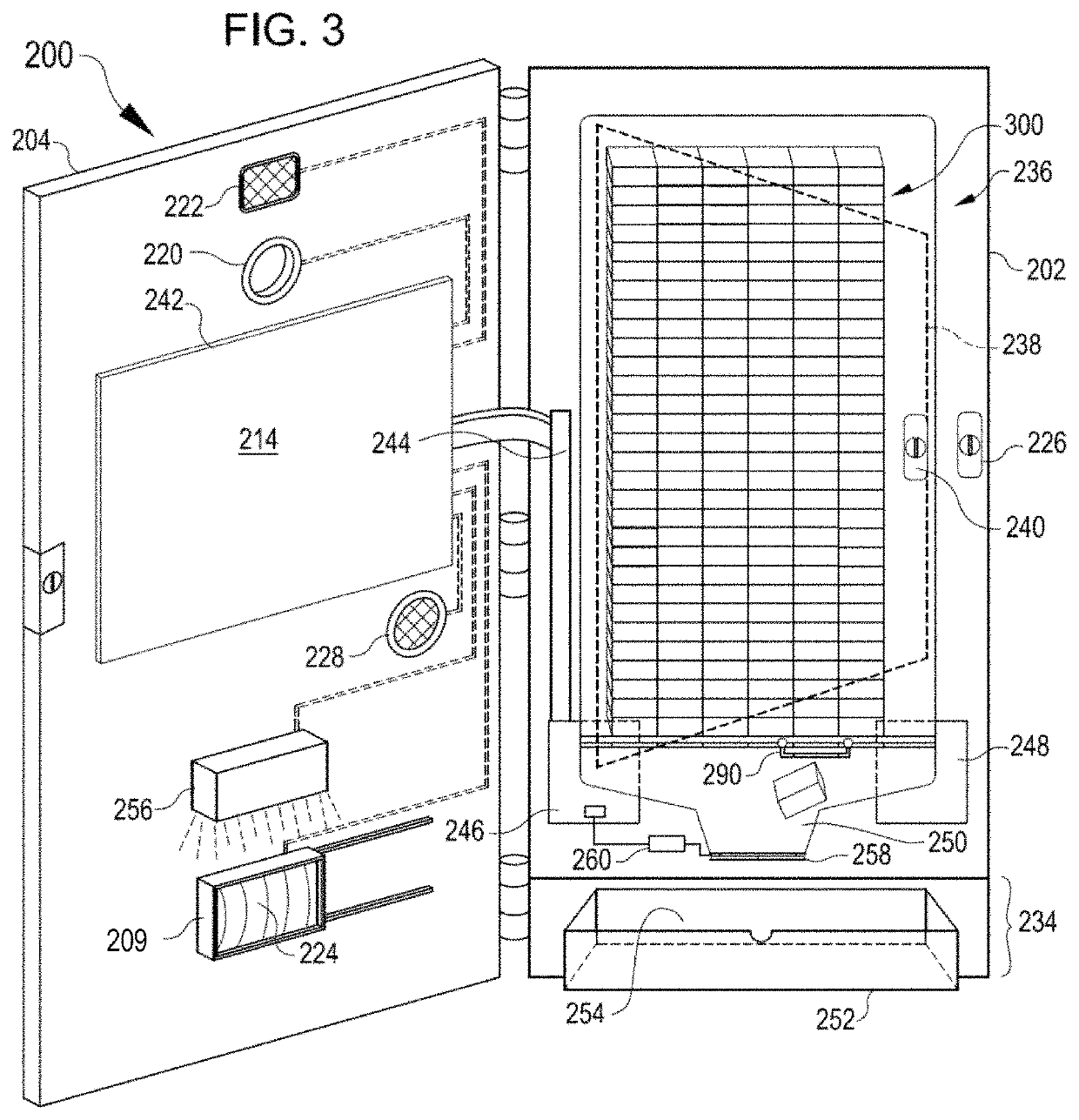
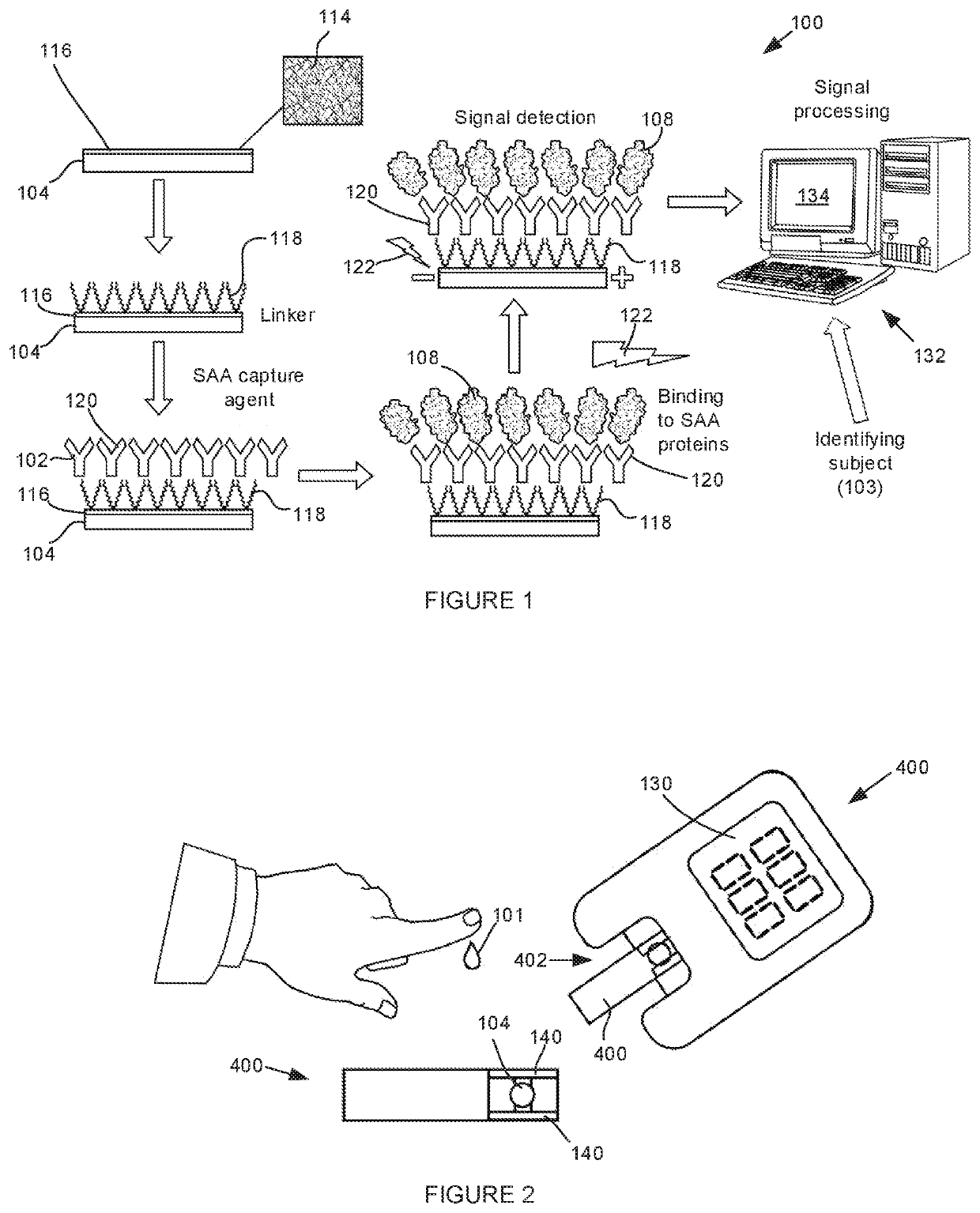
Methods, systems and devices for detecting inflammation
A method, system, test strip, point-of-care device and computer-implemented method for detecting a level of inflammation in a subject is provided. The level of inflammation is detected by contacting a biological sample obtained from the subject with a serum amyloid A (SAA) capture agent. The capture agent is secured to a substrate and is configured to emit a signal upon binding to SAA. The signal is detected and a result indicating the level of inflammation in the subject is output.
Owner:STELLENBOSCH UNIVERSITY
Light patterning of inorganic materials
PendingUS20220242992A1Polycrystalline material growthAdditive manufacturing apparatusChemical reactionElectromagnetic radiation
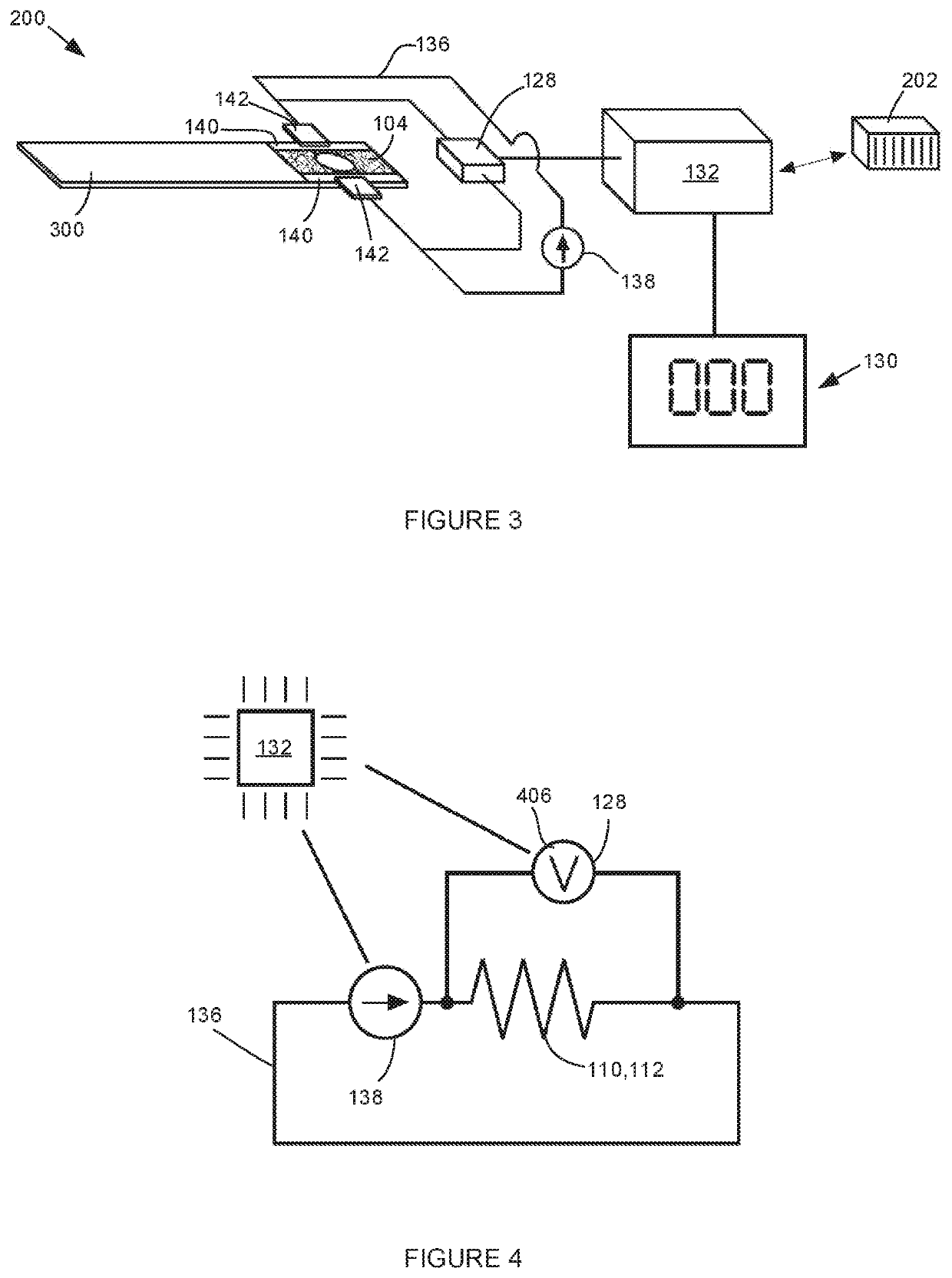
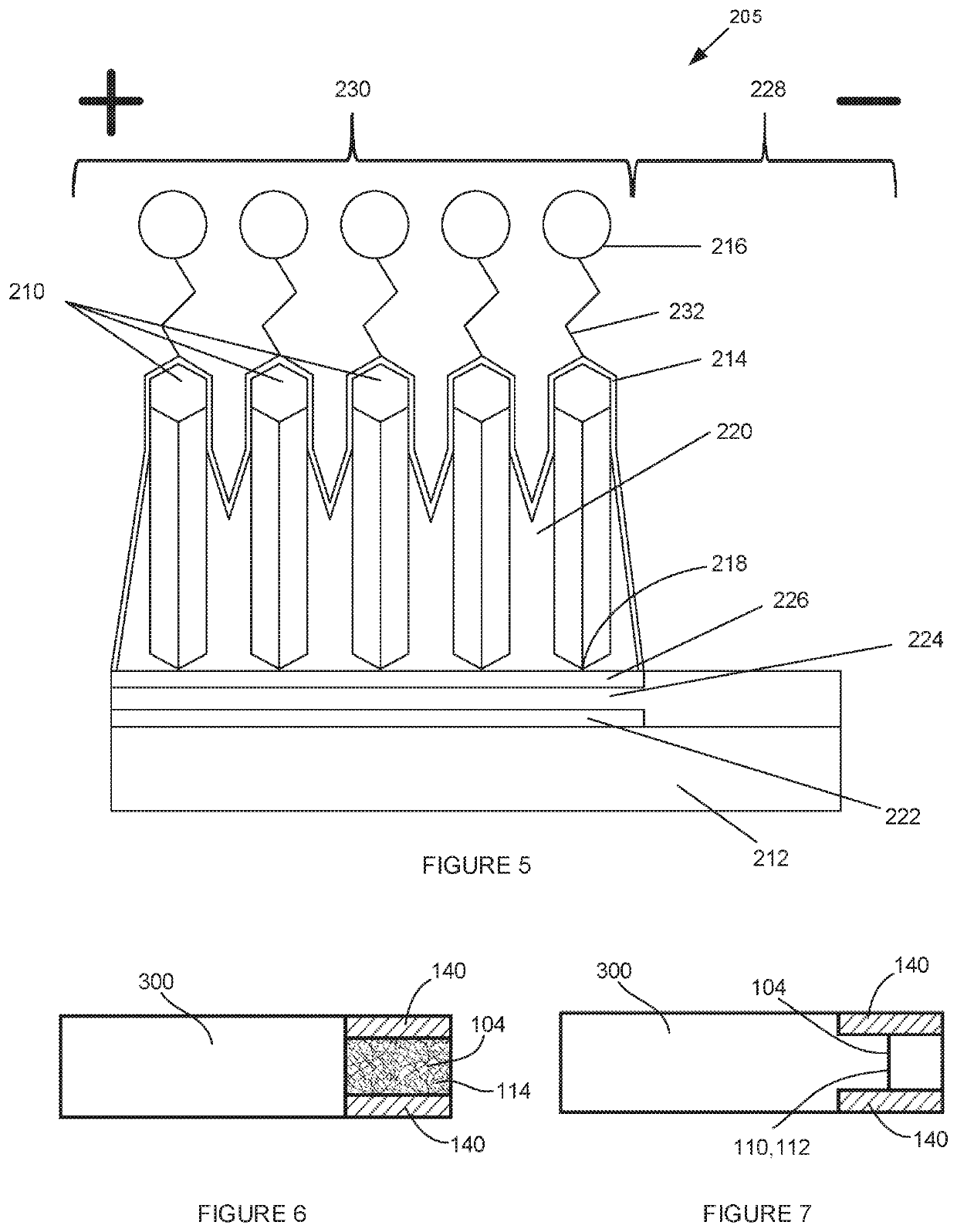
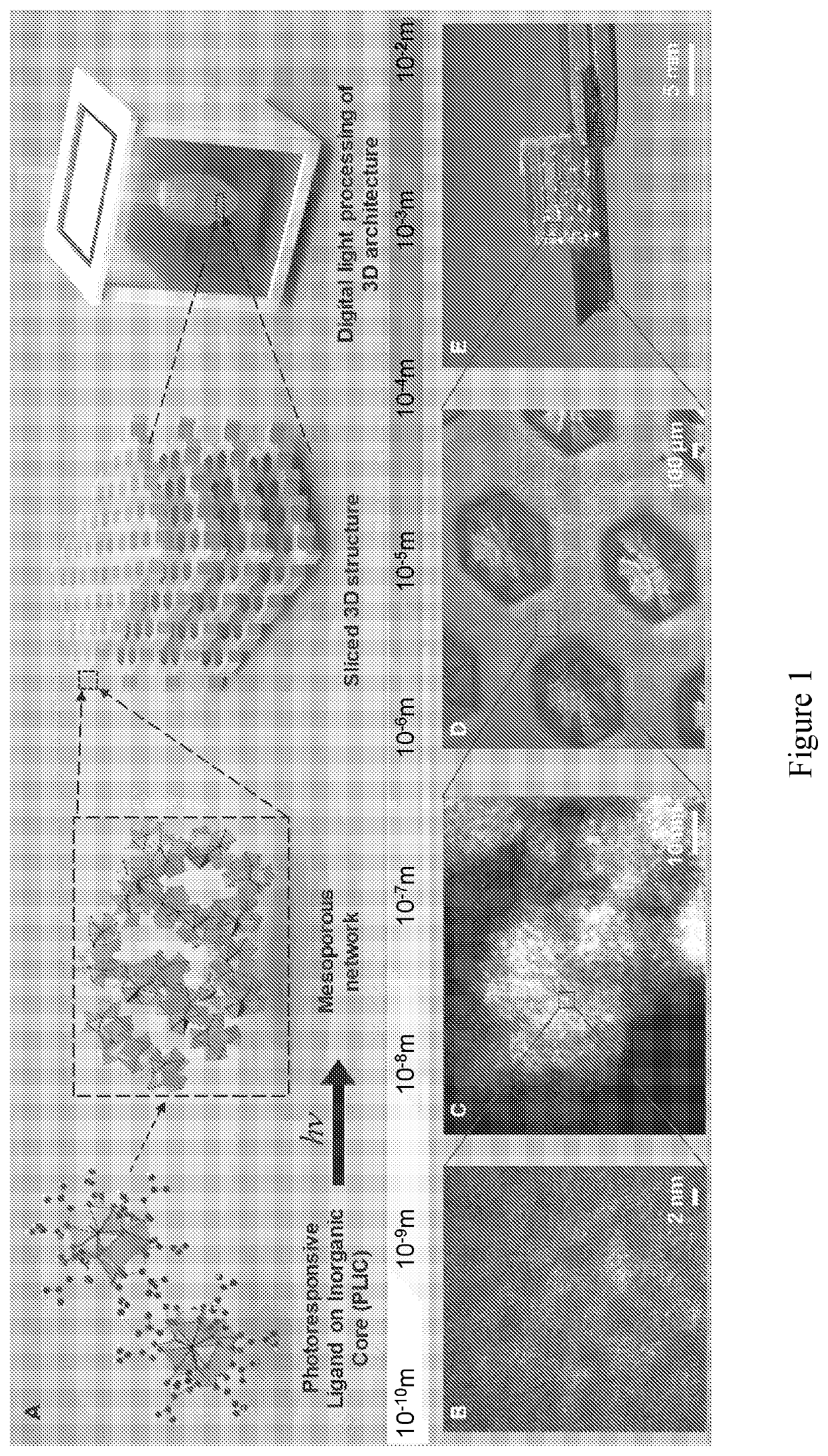
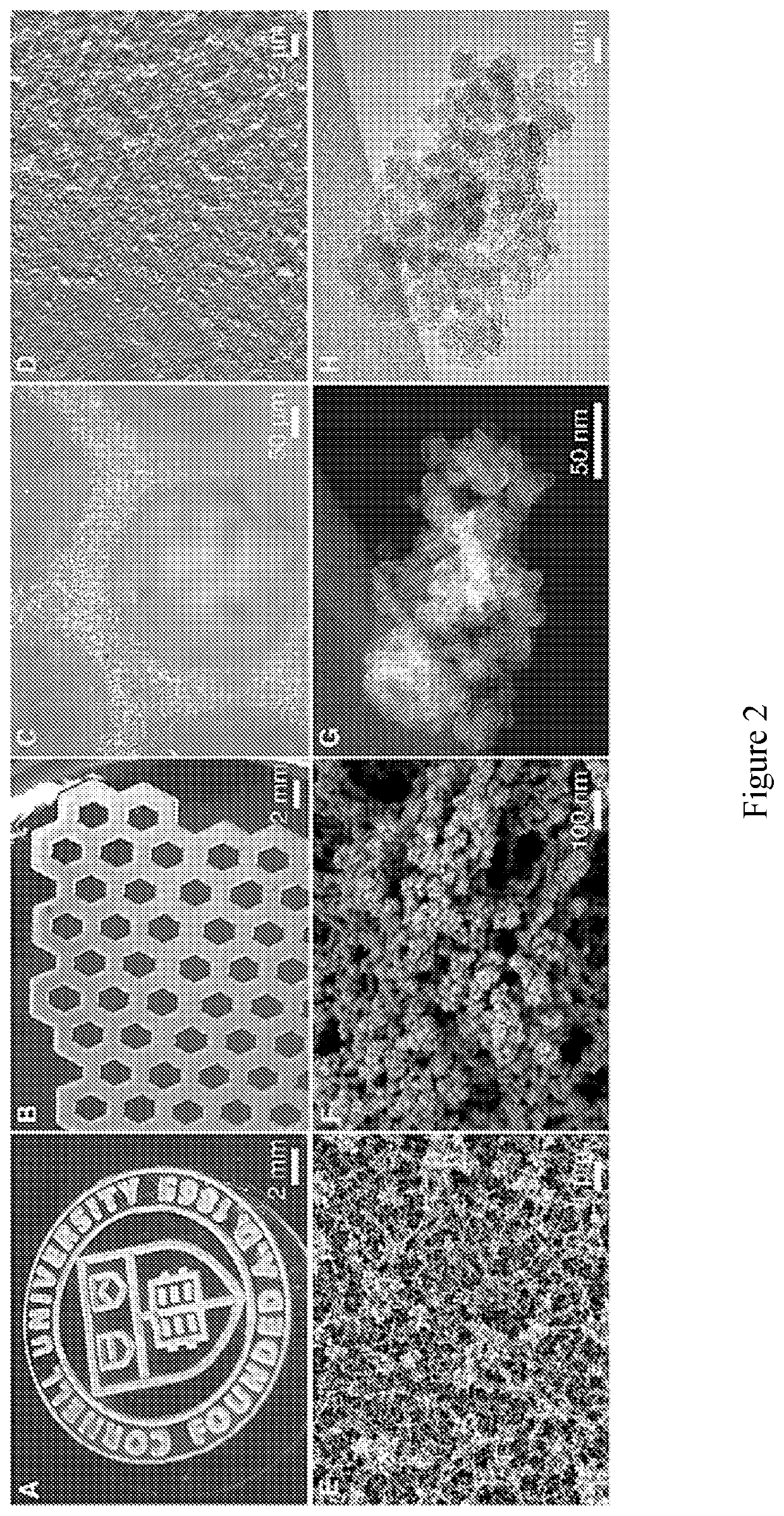
Compositions including a plurality of reactive components. The reactive components each include an inorganic core with one or more photoresponsive ligand(s) covalently bonded to a surface of the inorganic core. A composition may also include a photoinitiator. Methods of making an article of manufacture includes photochemically reacting at least a portion of one or more layer(s) formed from one or more composition(s). The photochemical reaction may be carried using a laser as a source of electromagnetic radiation. An article of manufacture, which may be a three-dimensional article of manufacture, may be or is a part of a microfluidic device, HPLC column, fluidic channel, point of care device, or diagnostics device.
Owner:CORNELL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com