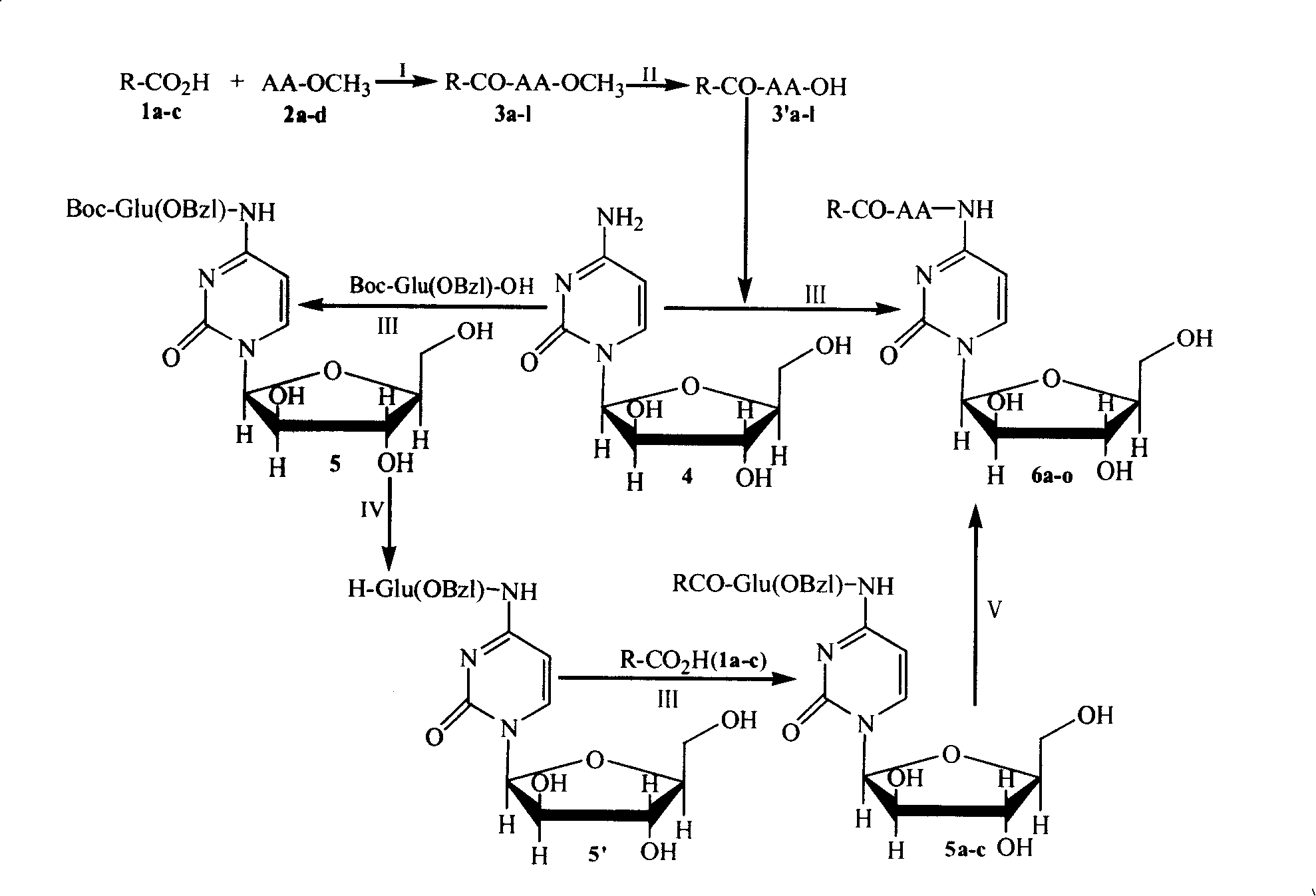
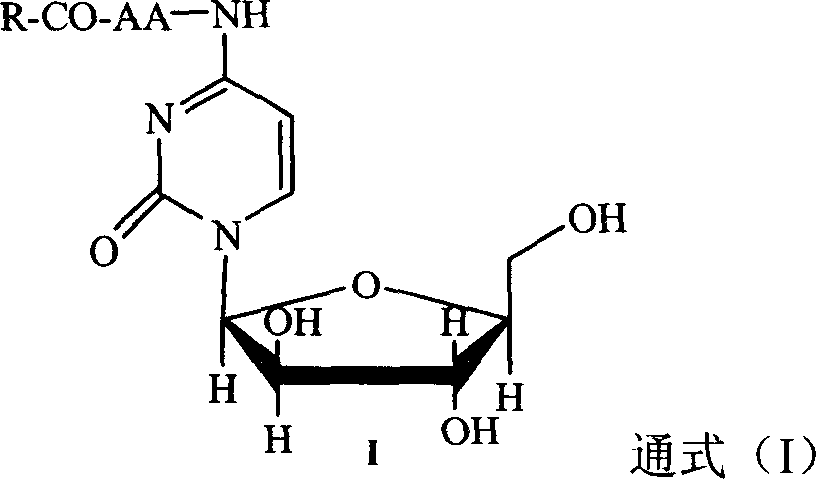
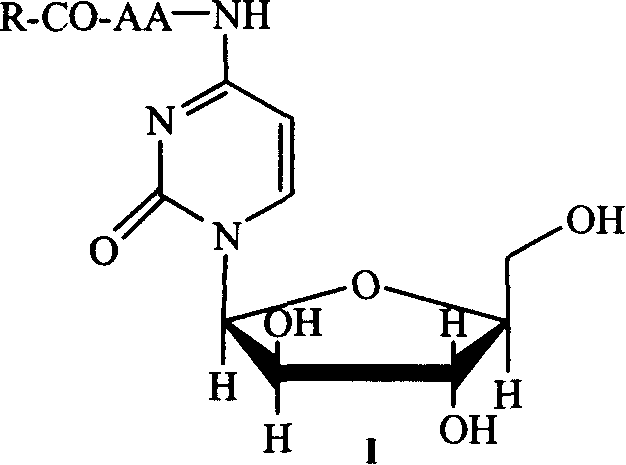
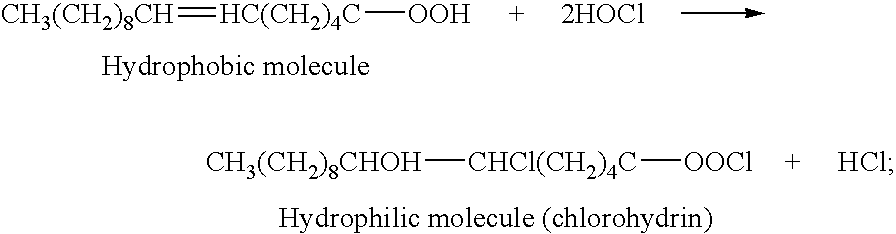Patents
Literature
230 results about "Drug vehicle" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Carrier or inert medium used as a solvent (or diluent) in which the medicinally active agent is formulated and or administered.
Foamable vehicle and pharmaceutical compositions thereof
A hygroscopic pharmaceutical composition includes at least one hygroscopic substance at a concentration sufficient to provide an Aw value of at least 0.9 and an antiinfective agent. A foamble pharmaceutical carrier includes about 50% to about 98% of a polar solvent selected from the group consisting of a polyol and PEG; 0% to about 48% of a secondary polar solvent; about 0.2% to about 5% by weight of a surface-active agent; about 0.01% to about 5% by weight of at least one polymeric agent; and a liquefied or compressed gas propellant at a concentration of about 3% to about 25% by weight of the total composition.
Owner:VYNE THERAPEUTICS INC
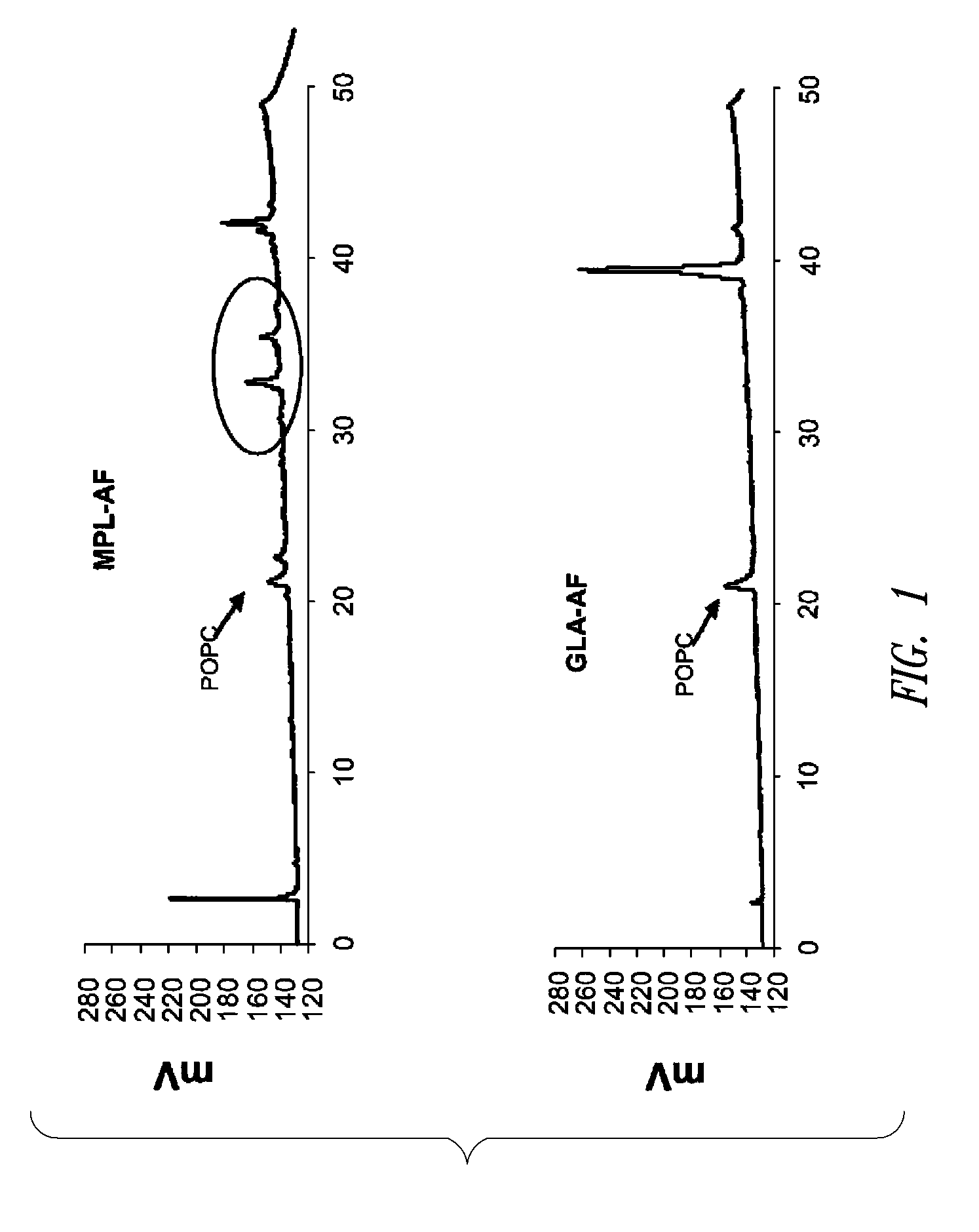
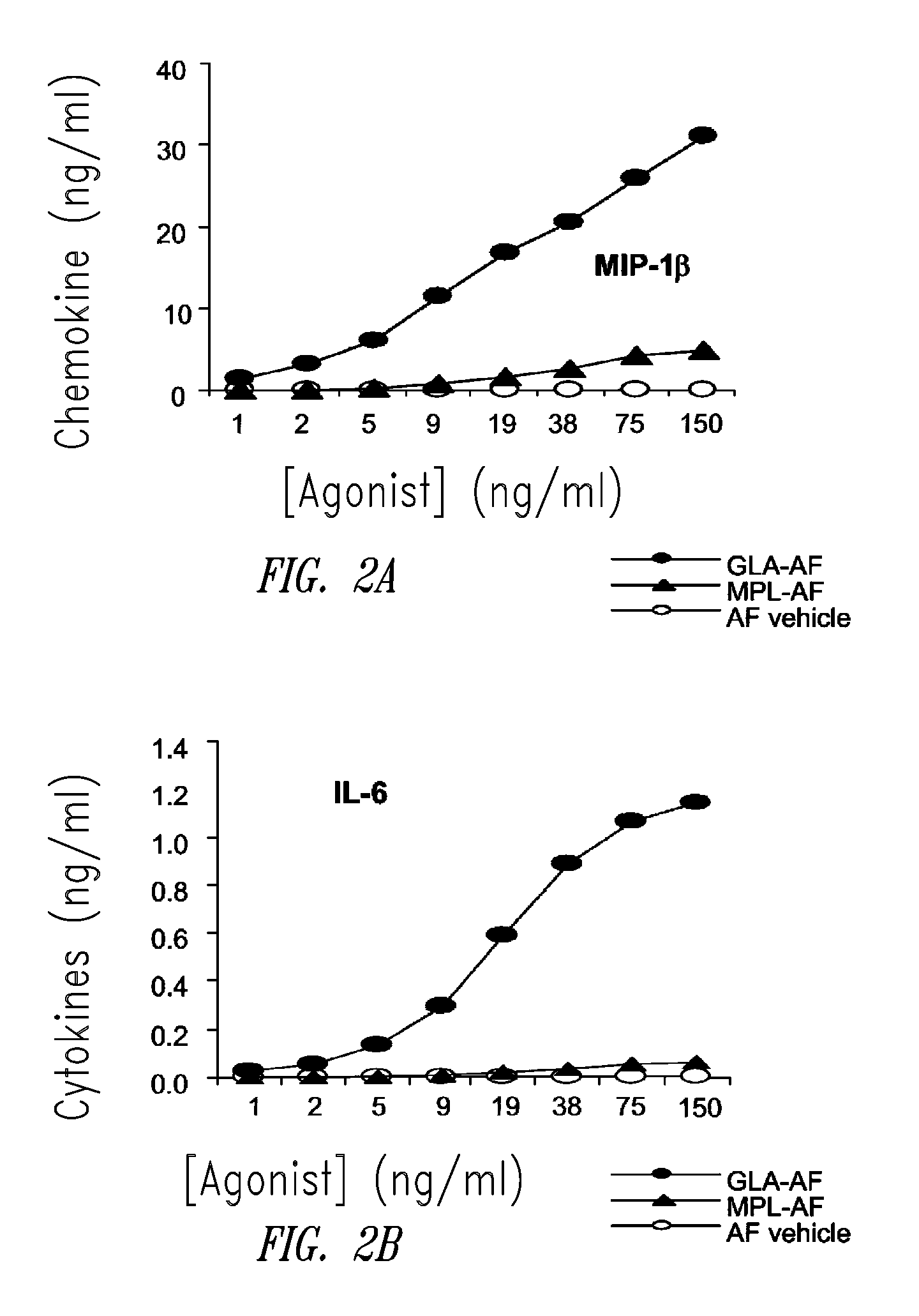
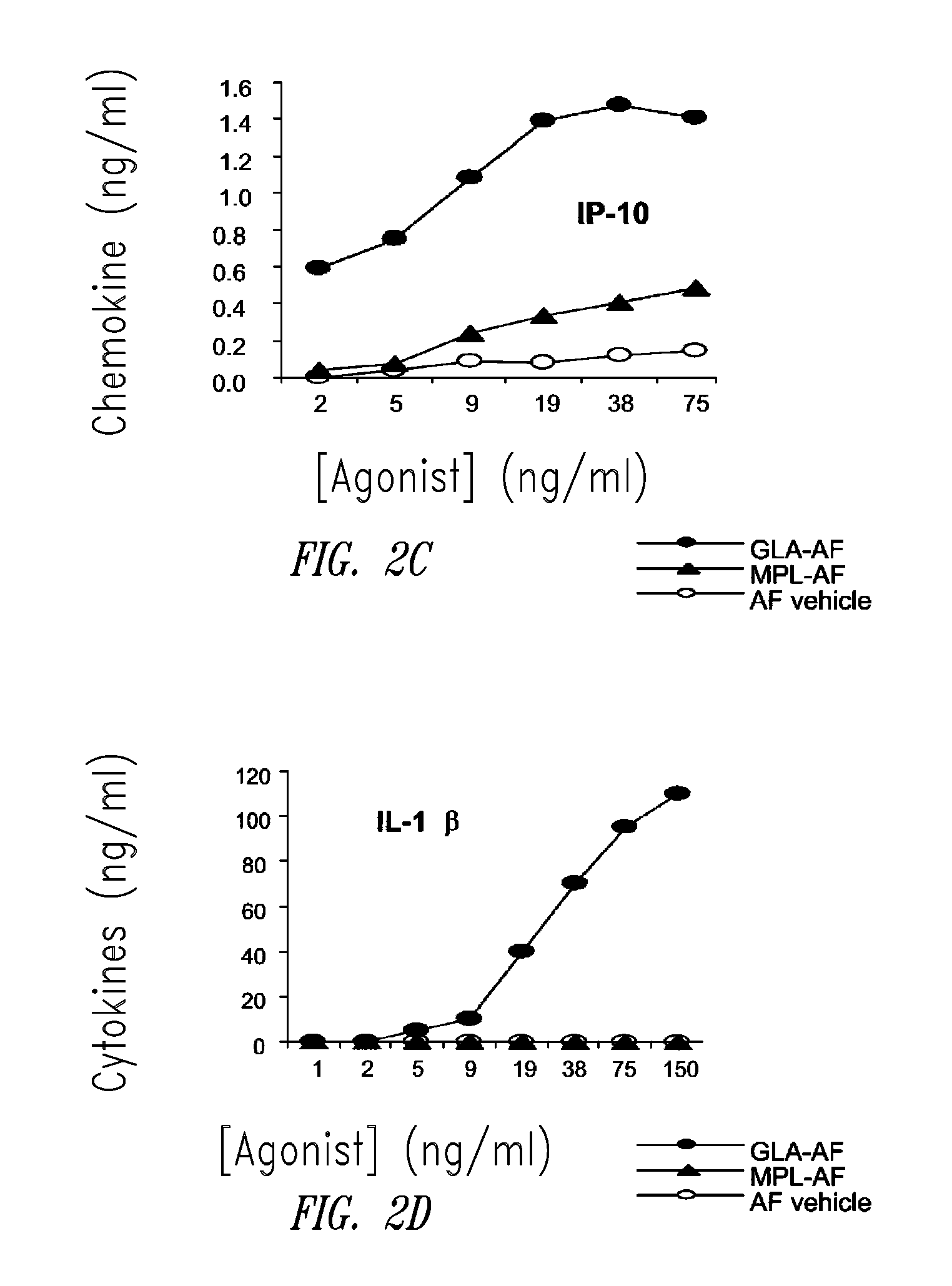
Vaccine composition containing synthetic adjuvant
ActiveUS20080131466A1Elicit immune responseAntibacterial agentsBacterial antigen ingredientsNatural productAdditive ingredient
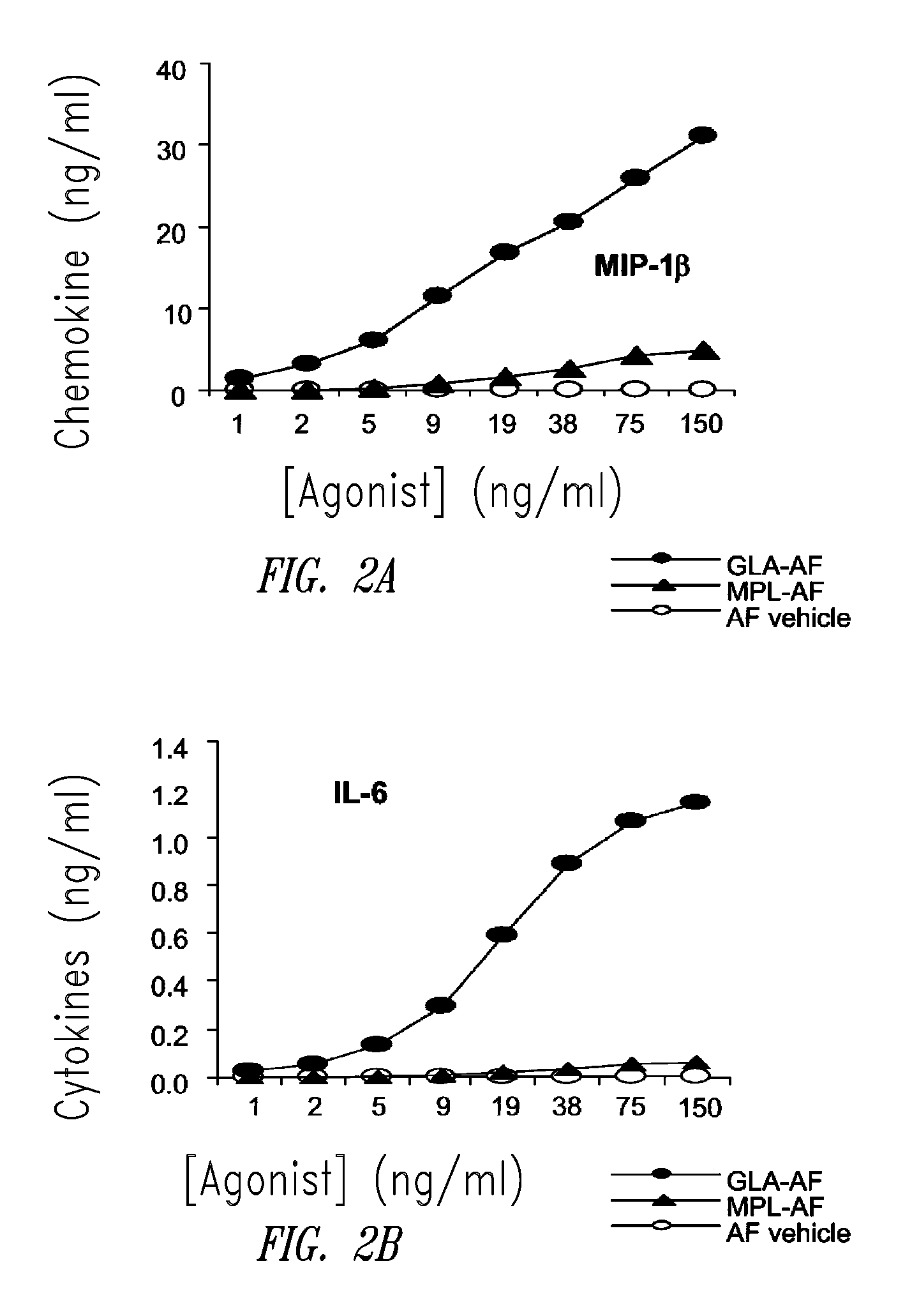
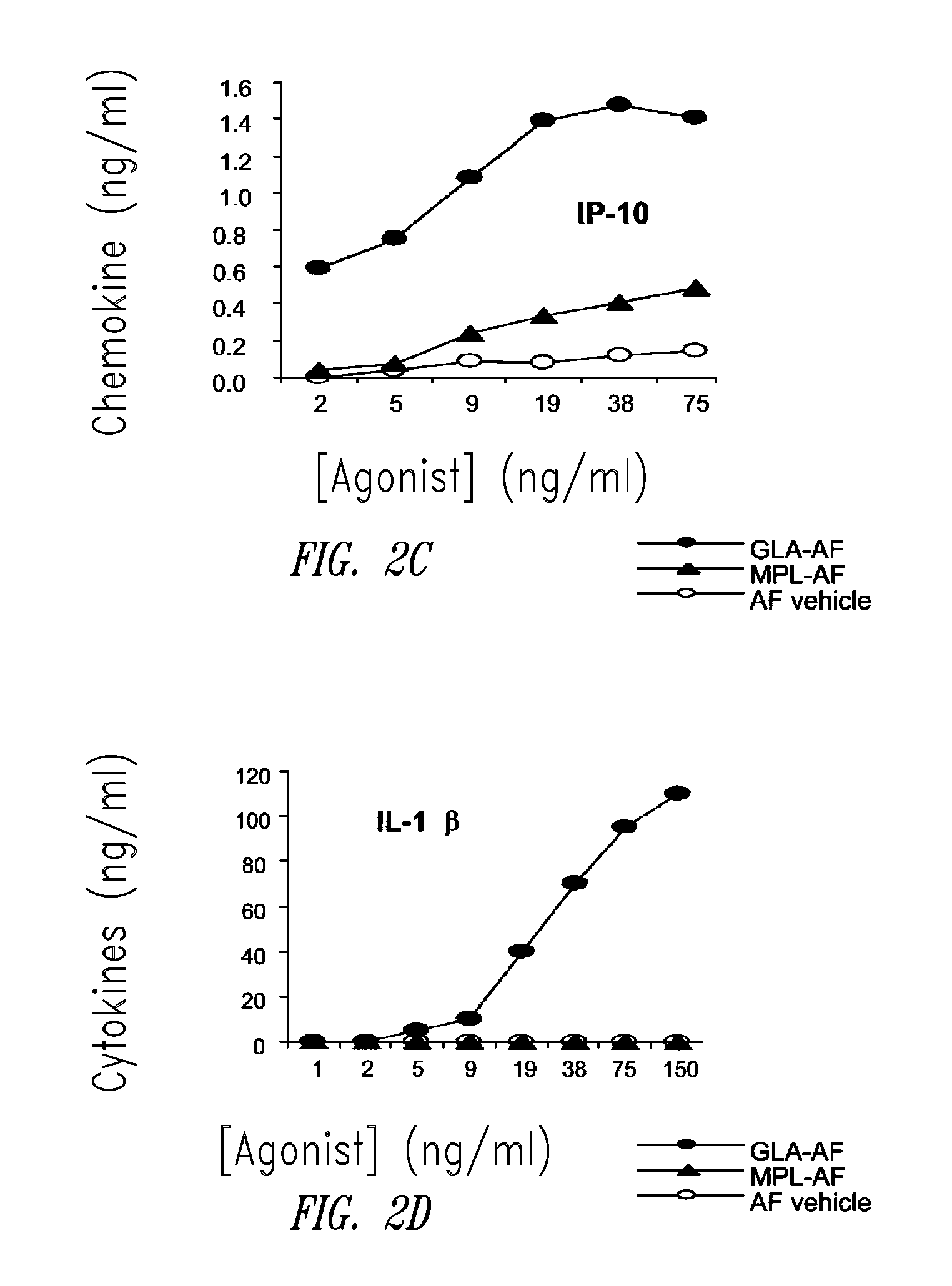
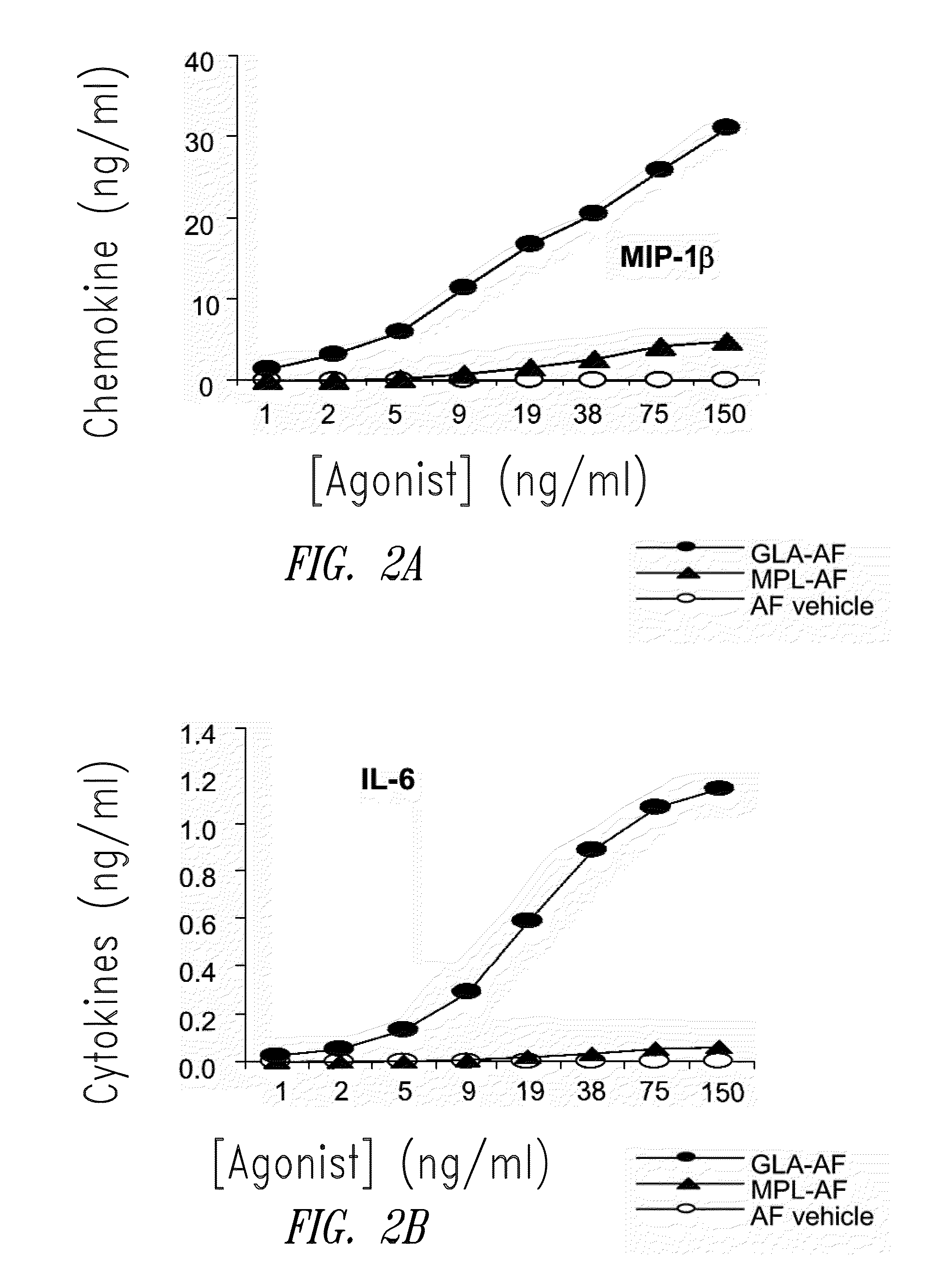
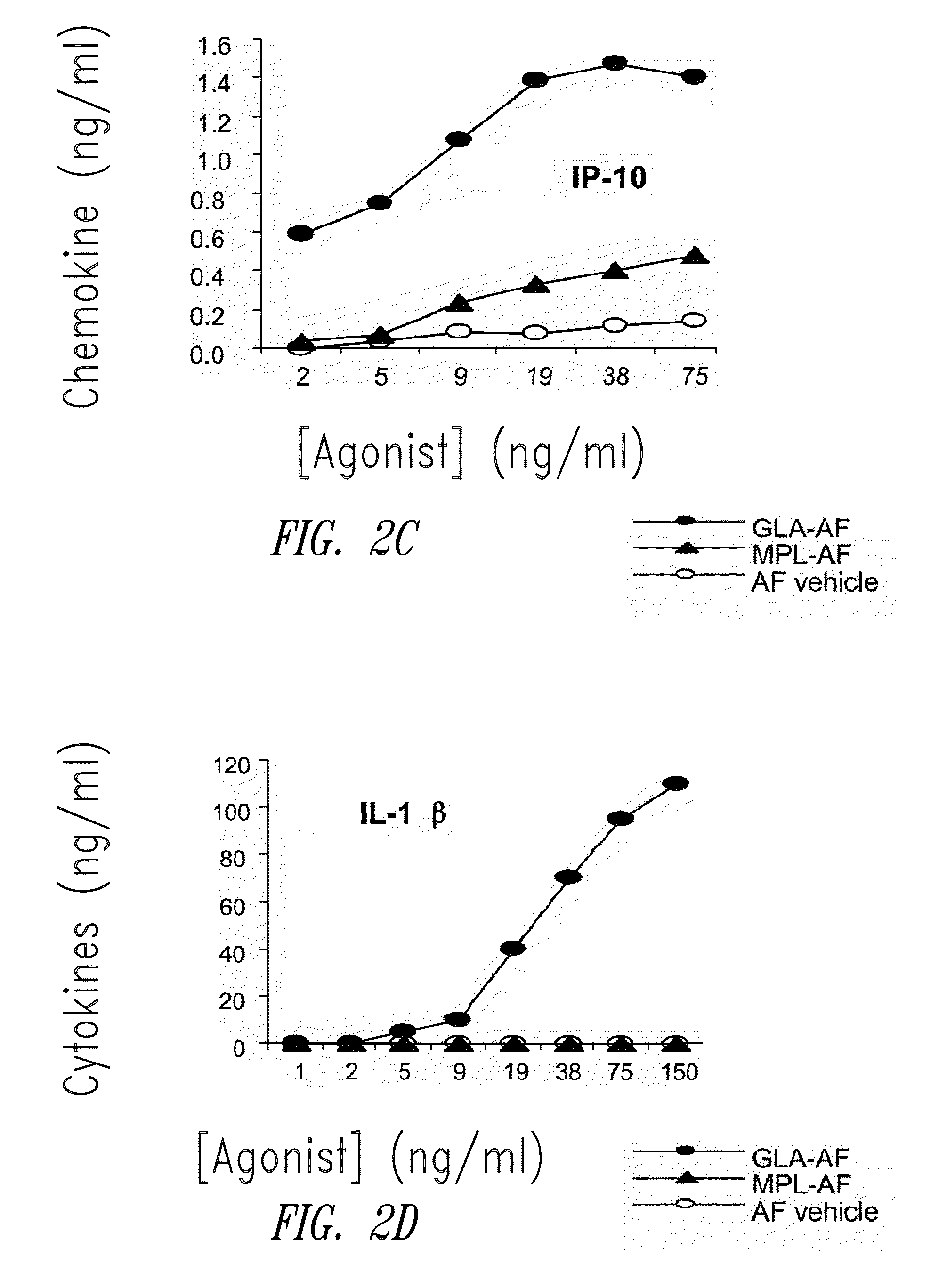
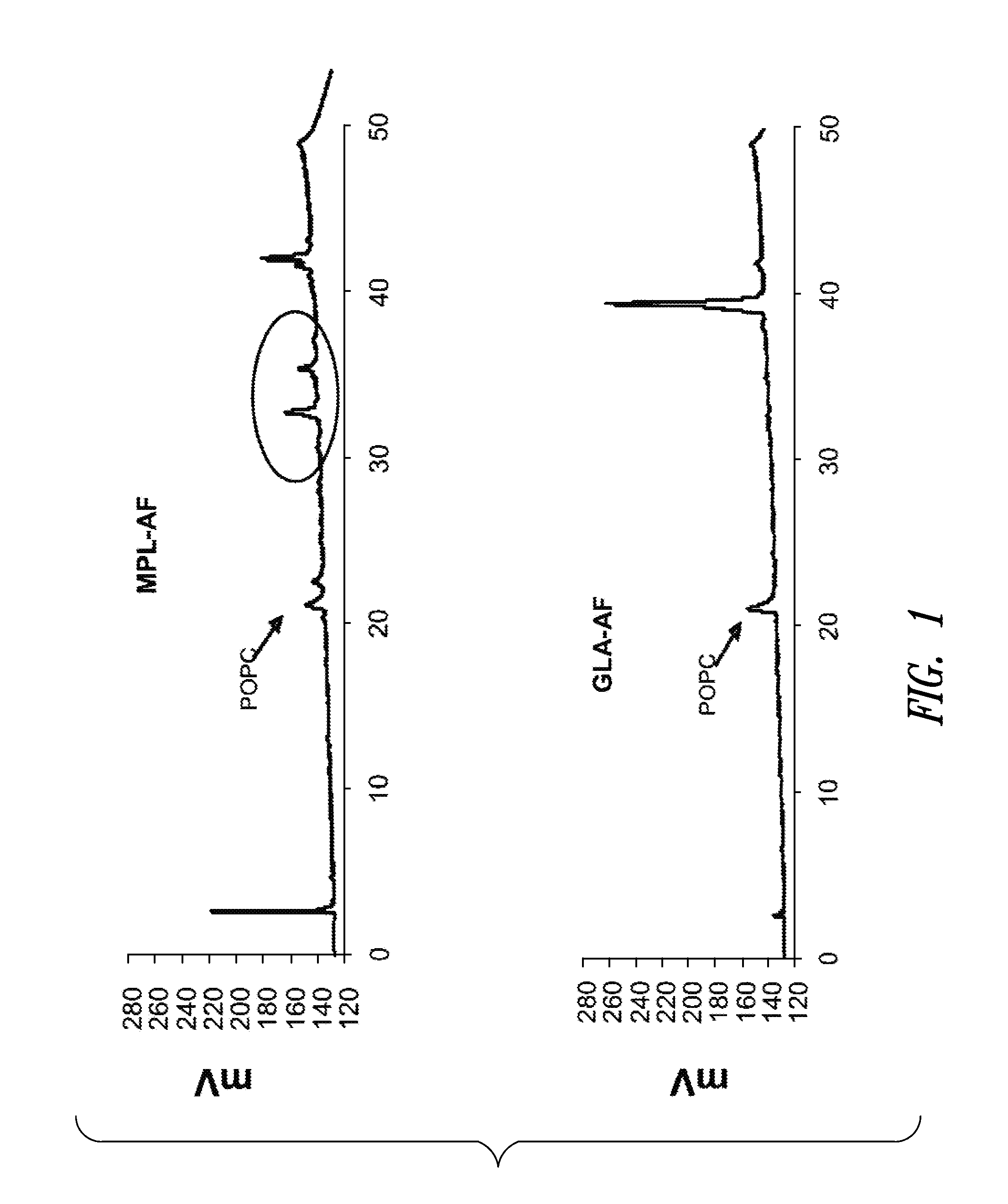
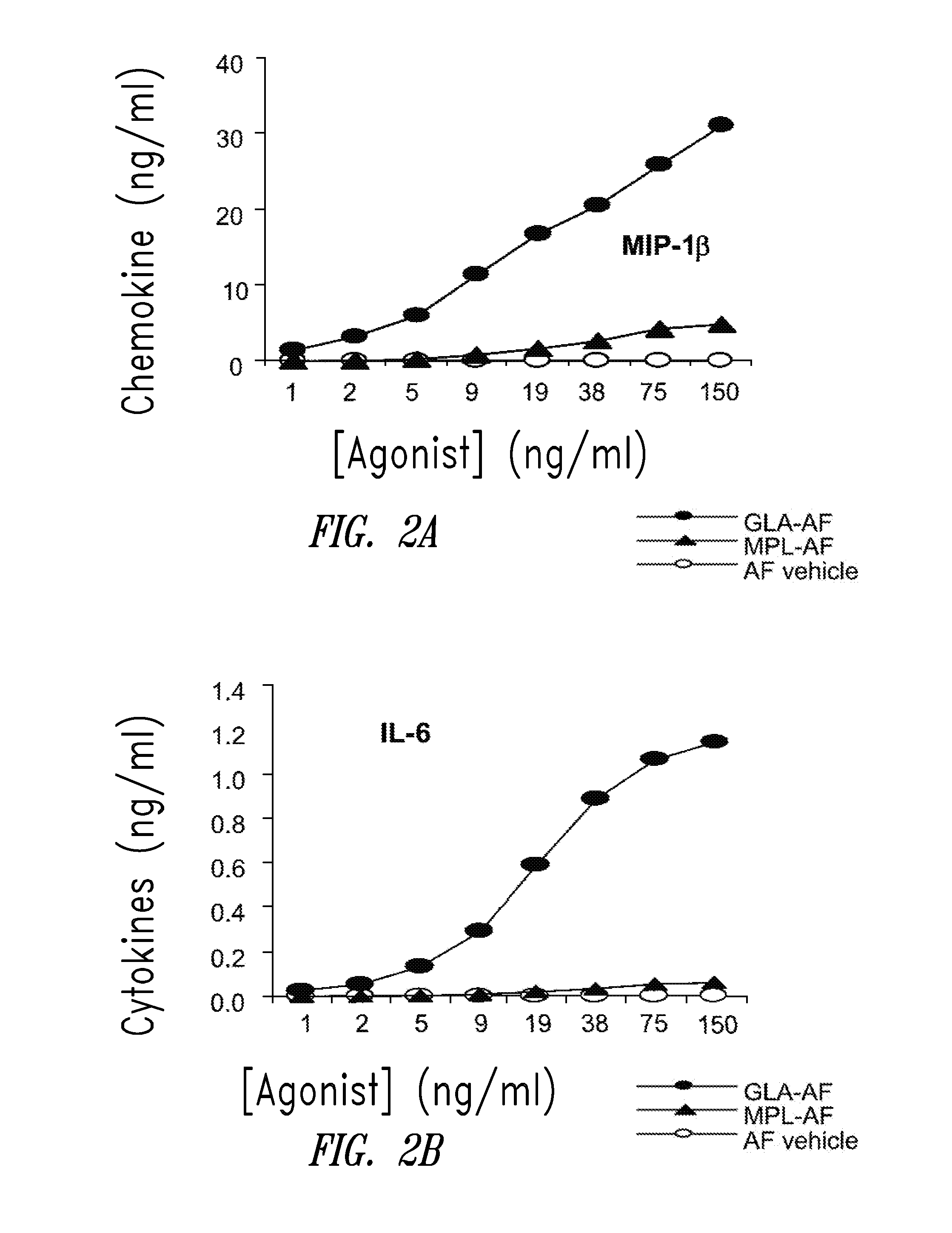
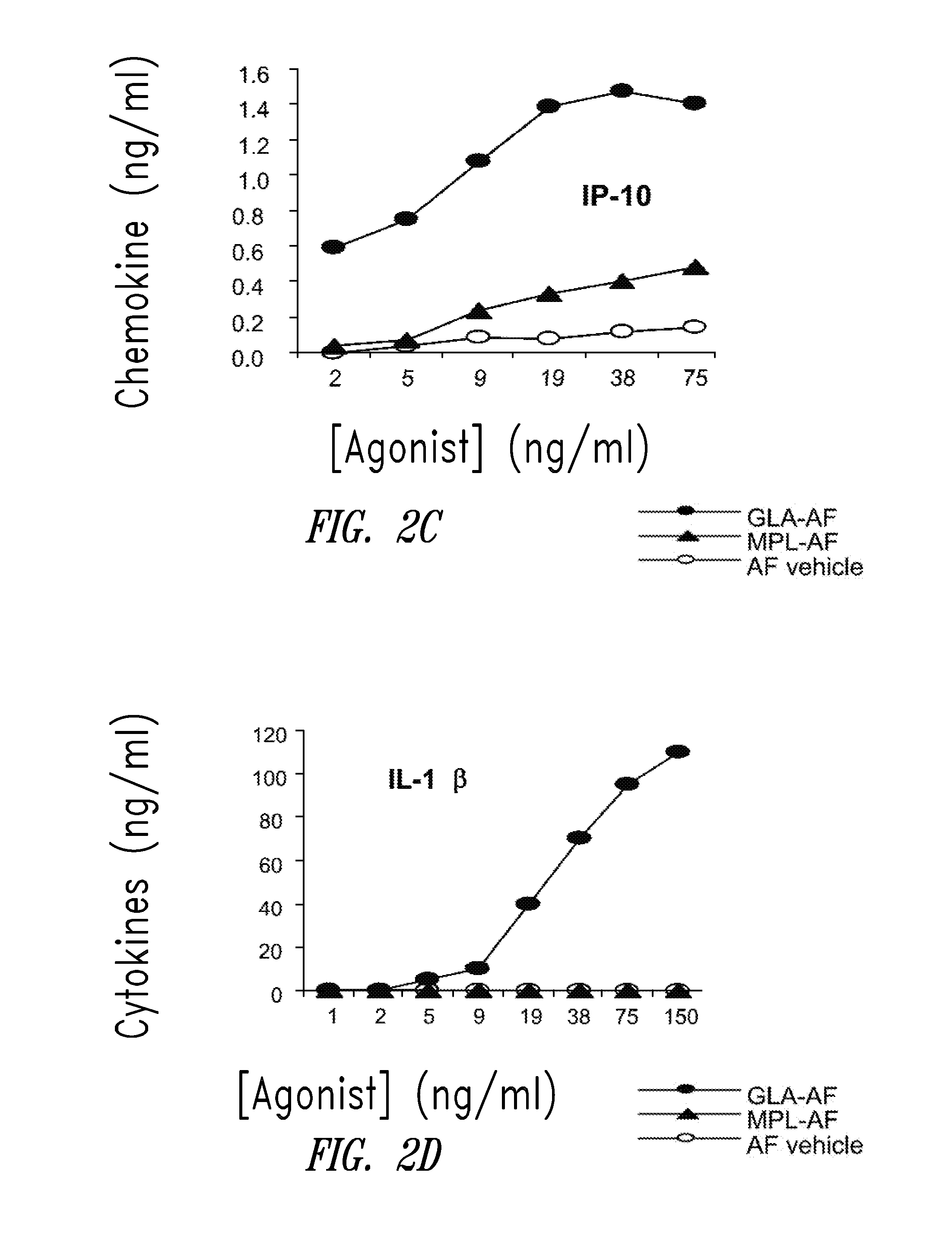
Compositions and methods, including vaccines and pharmaceutical compositions for inducing or enhancing an immune response are disclosed based on the discovery of useful immunological adjuvant properties in a synthetic, glucopyranosyl lipid adjuvant (GLA) that is provided in substantially homogeneous form. Chemically defined, synthetic GLA offers a consistent vaccine component from lot to lot without the fluctuations in contaminants or activity that compromise natural-product adjuvants. Also provided are vaccines and pharmaceutical compositions that include GLA and one or more of an antigen, a Toll-like receptor (TLR) agonist, a co-adjuvant and a carrier such as a pharmaceutical carrier.
Owner:ACCESS TO ADVANCED HEALTH INST
Polypropylene glycol foamable vehicle and pharmaceutical compositions thereof
The present invention teaches a foamable pharmaceutical carrier comprising polypropylene glycol (PPG) alkyl ether, a surface-active agent water and a liquefied hydrocarbon gas propellant; and pharmaceutical compositions thereof. The present invention further teaches a foamable pharmaceutical carrier comprising polypropylene glycol (PPG) alkyl ether, a surface-active agent, and a liquefied hydrocarbon gas propellant; and pharmaceutical compositions thereof.
Owner:FOAMIX PHARMACEUTICALS LIMITED
Dicarboxylic acid foamable vehicle and pharmaceutical compositions thereof
ActiveUS20080044444A1Convenient vehicle for topical deliveryGood treatment effectPowder deliveryBiocideDicarboxylic acidCarboxylic acid
The present invention teaches a foamable pharmaceutical carrier comprising a benefit agent, selected from the group consisting of a dicarboxylic acid and a dicarboxylic acid ester; a stabilizer selected from the group consisting of at least one surface-active agent; at least one polymeric agent and mixtures thereof; a solvent selected from the group consisting of water, a hydrophilic solvent, a hydrophobic solvent, a potent solvent, a polar solvent, a silicone, an emollient, and mixtures thereof, wherein the benefit agent, stabilizer and solvent are selected to provide a composition that is substantially resistant to aging and to phase separation and or can substantially stabilize other active ingredients. The invention further relates to a foamable composition further containing a liquefied hydrocarbon gas propellant.
Owner:VYNE THERAPEUTICS INC
Foamable vehicle and pharmaceutical compositions thereof
Owner:VYNE THERAPEUTICS INC
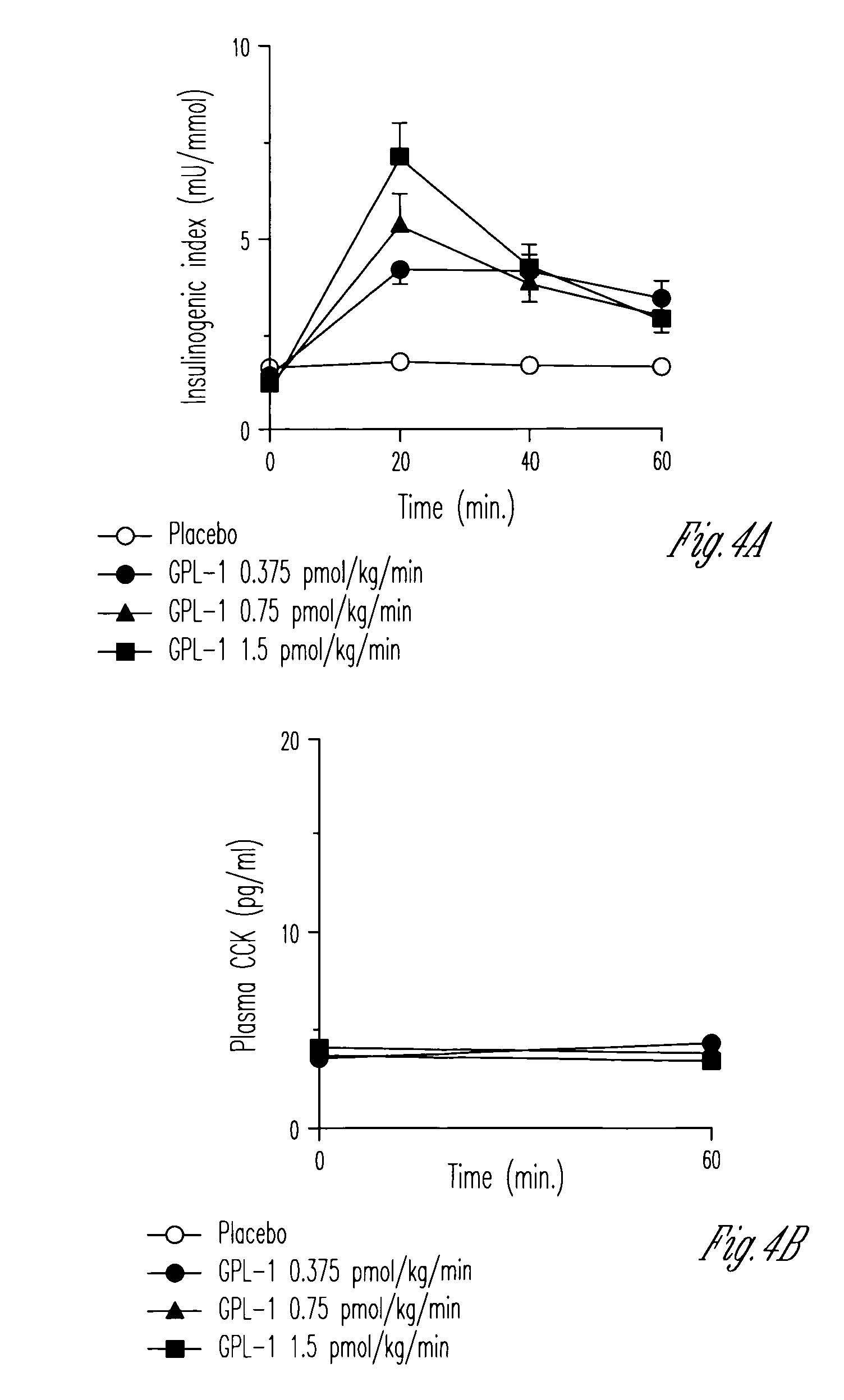
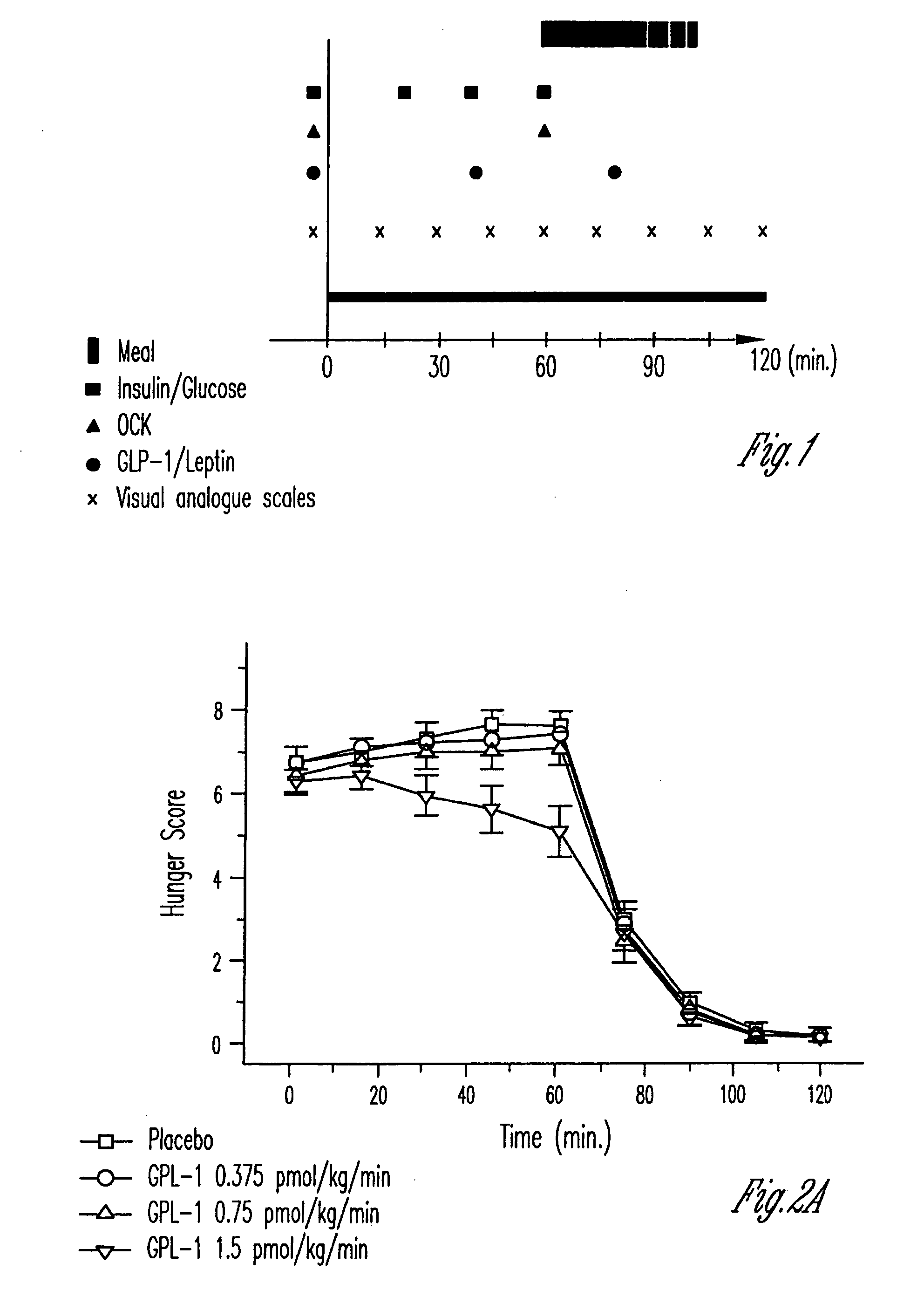
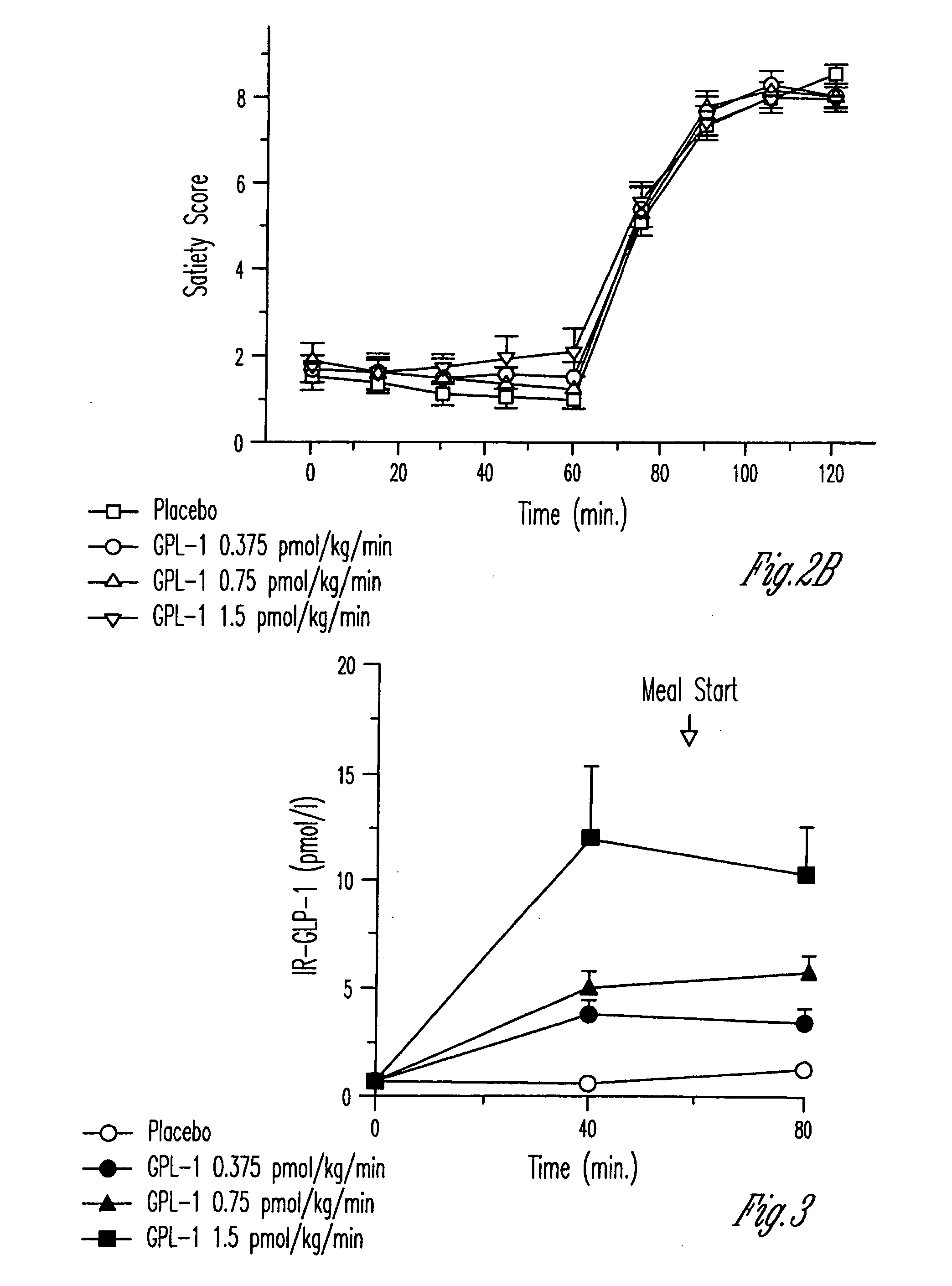
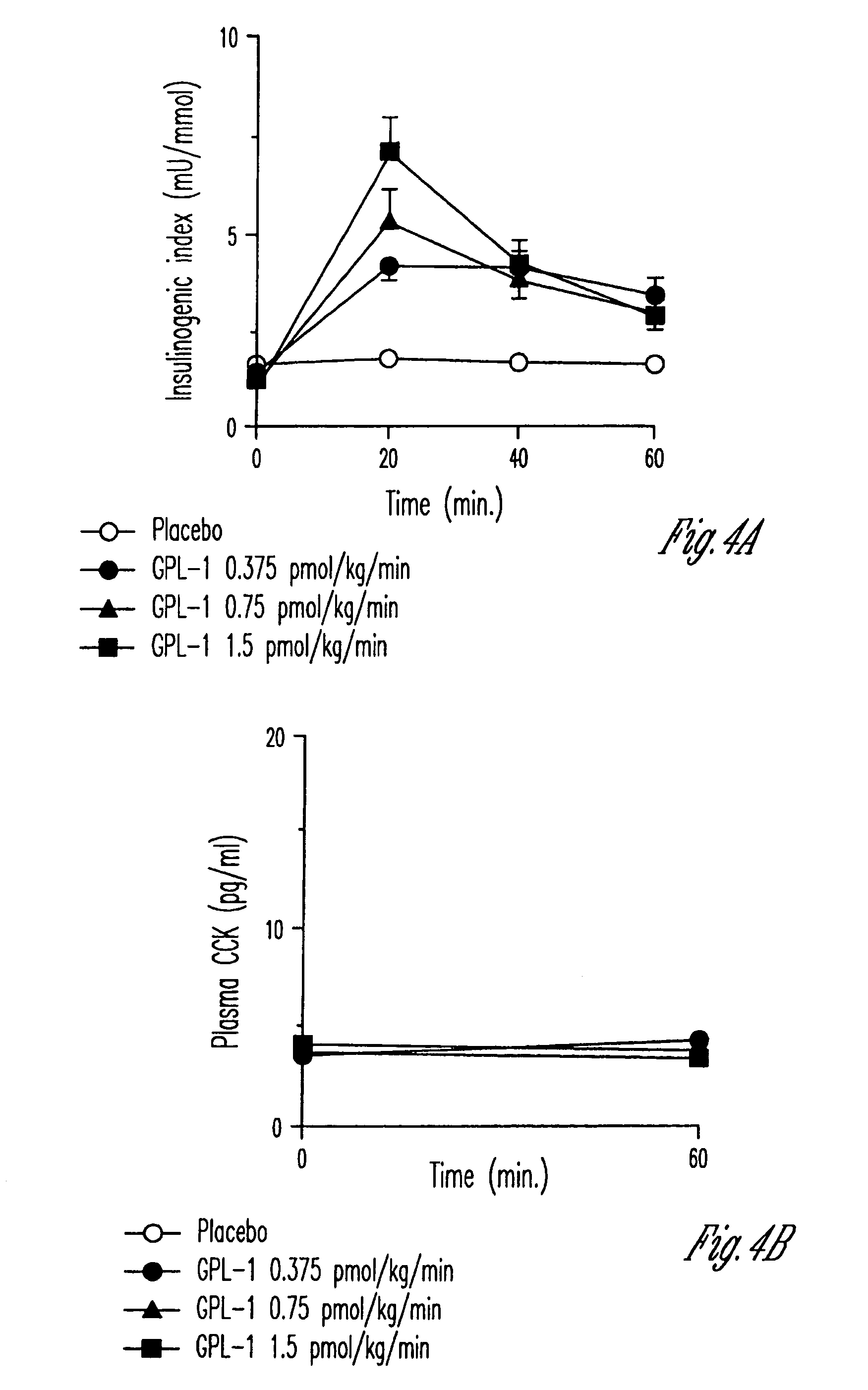
Human appetite control by glucagon-like peptide receptor binding compounds
InactiveUS6998387B1Appetite suppressantReducing spontaneous food intakePeptide/protein ingredientsMetabolism disorderCompound aGlucagon-like peptide-1
A composition including a compound which binds to a receptor for glucagon-like peptide-1 and a pharmaceutical carrier. The amount of the compound present is effective to control appetite in a human. Also disclosed is a method for controlling appetite and for reducing food intake in a human by administering to the human a composition comprising a compound which binds to a receptor for glucagon-like peptide-1 and a pharmaceutical carrier.
Owner:ASTRAZENECA PHARMA LP
Polypropylene glycol foamable vehicle and pharmaceutical compositions thereof
InactiveUS20080152596A1Easy to useCosmetic preparationsBiocidePolypropylene glycolSurface-active agents
The present invention teaches a foamable pharmaceutical carrier comprising polypropylene glycol (PPG) alkyl ether, a surface-active agent water and a liquefied hydrocarbon gas propellant; and pharmaceutical compositions thereof.The present invention further teaches a foamable pharmaceutical carrier comprising polypropylene glycol (PPG) alkyl ether, a surface-active agent, and a liquefied hydrocarbon gas propellant; and pharmaceutical compositions thereof.
Owner:FOAMIX PHARMACEUTICALS LIMITED
Vaccine composition containing synthetic adjuvant
InactiveUS20090181078A1Promote maturitySsRNA viruses negative-senseVertebrate antigen ingredientsNatural productPharmaceutical drug
Compositions and methods, including vaccines and pharmaceutical compositions for inducing or enhancing an immune response are disclosed based on the discovery of useful immunological adjuvant properties in a synthetic, glucopyranosyl lipid adjuvant (GLA) that is provided in substantially homogeneous form. Chemically defined, synthetic GLA offers a consistent vaccine component from lot to lot without the fluctuations in contaminants or activity that compromise natural-product adjuvants. Also provided are vaccines and pharmaceutical compositions that include GLA and one or more of an antigen, a Toll-like receptor (TLR) agonist, a co-adjuvant and a carrier such as a pharmaceutical carrier.
Owner:INFECTIOUS DISEASE RES INST
Dicarboxylic acid foamable vehicle and pharmaceutical compositions thereof
The present invention teaches a foamable pharmaceutical carrier comprising a benefit agent, selected from the group consisting of a dicarboxylic acid and a dicarboxylic acid ester; a stabilizer selected from the group consisting of at least one surface-active agent; at least one polymeric agent and mixtures thereof; a solvent selected from the group consisting of water, a hydrophilic solvent, a hydrophobic solvent, a potent solvent, a polar solvent, a silicone, an emollient, and mixtures thereof, wherein the benefit agent, stabilizer and solvent are selected to provide a composition that is substantially resistant to aging and to phase separation and or can substantially stabilize other active ingredients. The invention further relates to a foamable composition further containing a liquefied hydrocarbon gas propellant.
Owner:FOAMIX PHARMACEUTICALS LIMITED
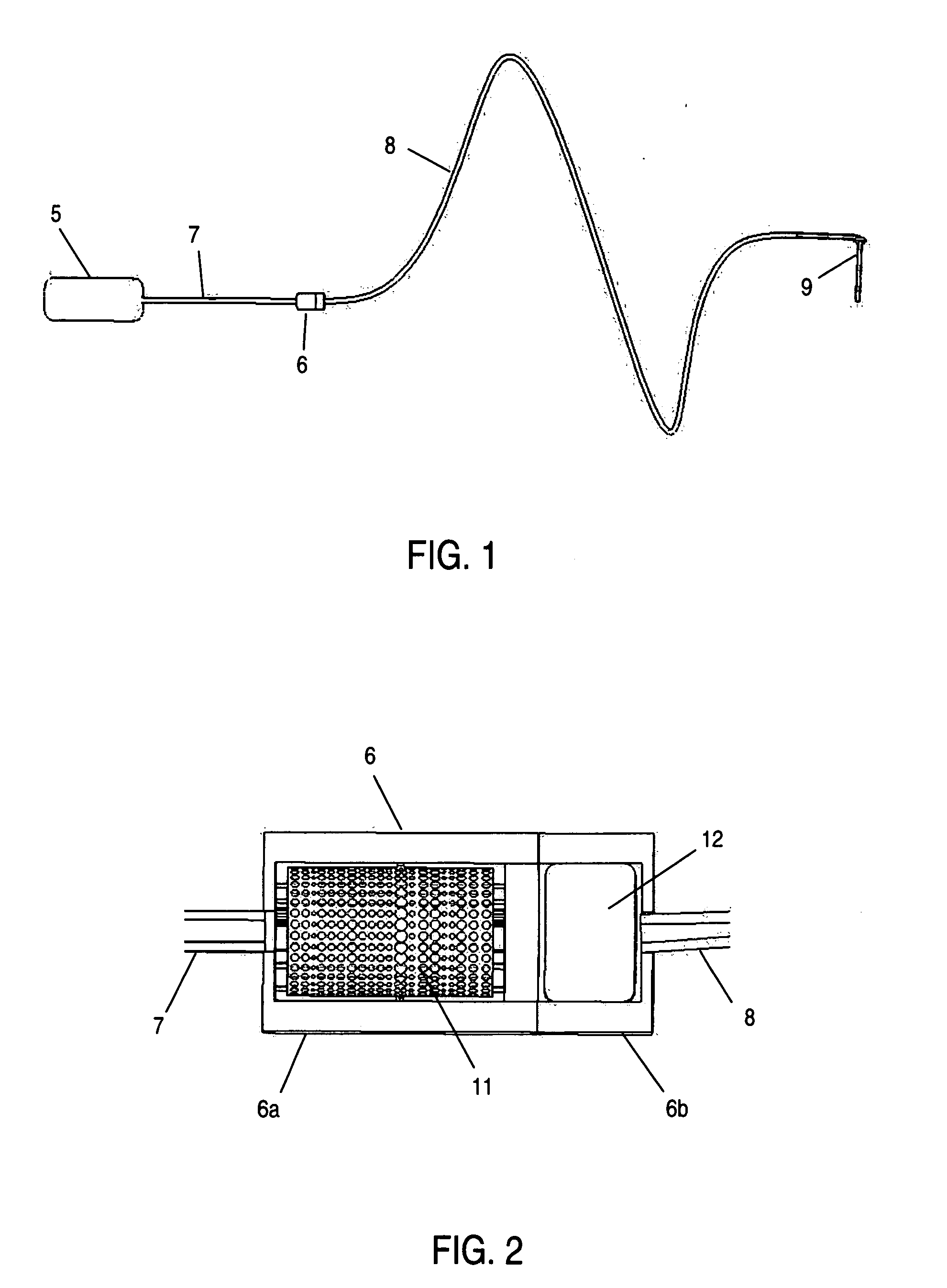
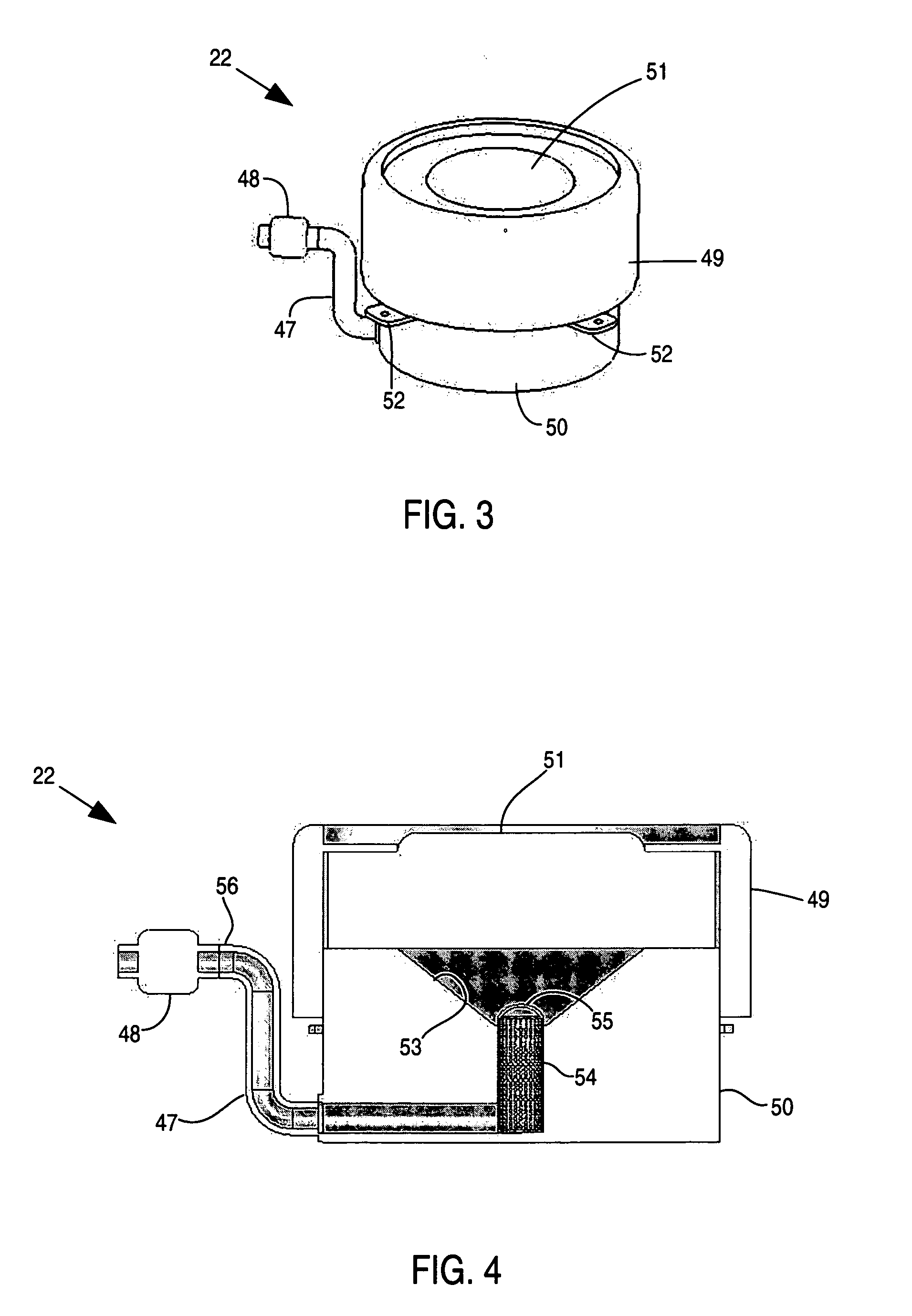
Apparatus and method for delivery of therapeutic and other types of agents
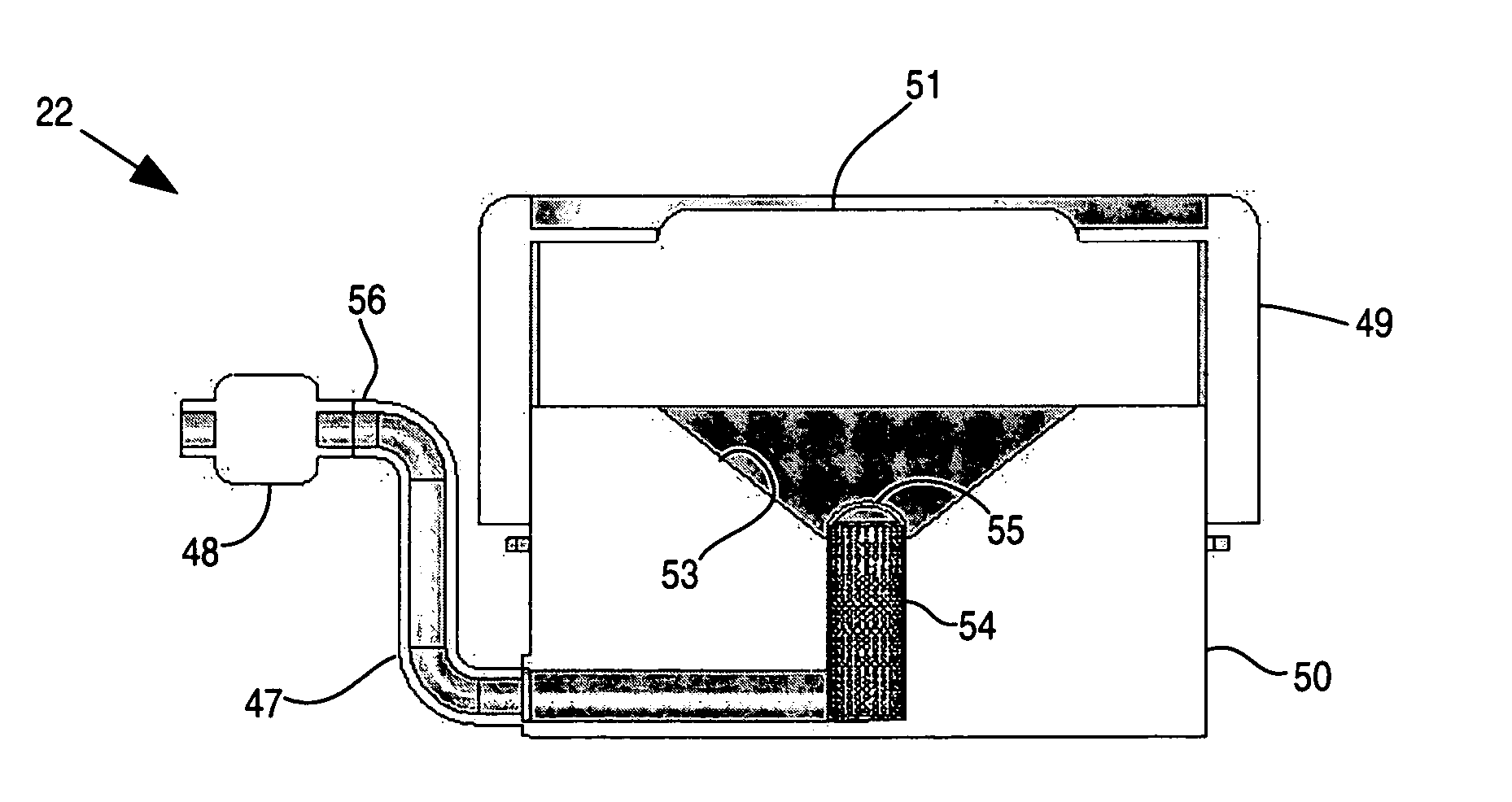
InactiveUS20070255237A1Convenient treatmentHead electrodesFiltering accessoriesOsmotic pumpTarget tissue
Implantable drug delivery systems target delivery of small volumes of drugs to specific tissues. In some cases, a drug delivery system includes an implantable osmotic pump connected to a drug-containing housing, with that housing connected to a needle, cochlear implant or other type of component for ultimate delivery to the target tissue. In some implementations, a subcutaneous port receives a fluid from an external pump. The port is connected to a needle or other component for delivery of one or more drugs to the target tissue. Both solid and liquid drug formulations can be used. In embodiments using solid drugs, a separate drug vehicle (such as saline) can be used to dissolve a portion of the solid drug, with the drug-loaded vehicle then delivered to the target tissue.
Owner:OTONOMY INC
Dicarboxylic acid foamable vehicle and pharmaceutical compositions thereof
The present invention teaches a foamable pharmaceutical carrier comprising a benefit agent, selected from the group consisting of a dicarboxylic acid and a dicarboxylic acid ester; a stabilizer selected from the group consisting of at least one surface-active agent; at least one polymeric agent and mixtures thereof; a solvent selected from the group consisting of water, a hydrophilic solvent, a hydrophobic solvent, a potent solvent, a polar solvent, a silicone, an emollient, and mixtures thereof, wherein the benefit agent, stabilizer and solvent are selected to provide a composition that is substantially resistant to aging and to phase separation and or can substantially stabilize other active ingredients. The invention further relates to a foamable composition further containing a liquefied hydrocarbon gas propellant.
Owner:VYNE THERAPEUTICS INC
Vaccine composition containing synthetic adjuvant
Owner:ACCESS TO ADVANCED HEALTH INST
Preparation method of porous chitosan-based microspheres
ActiveCN104722251AUniform particle sizeRich sourcesOther chemical processesMicroballoon preparationWound dressingEmulsion
The invention relates to the field of high-molecular porous materials and particularly relates to a preparation method of porous chitosan-based microspheres for medicine carriers, adsorption and separation, hemostasis and wound dressing. The preparation method comprises the following steps: (a) respectively preparing solidification liquid, an emsulsifying-agent solution and a diluted-acid solution of pelletizing materials and precooling the solidification solution to minus 20 DEG C; (b) dripping the chitosan diluted-acid solution into the emsulsifying-agent solution, homogenizing and stirring to form emulsion; and (c) carrying out thermally-induced phase separation on the emulsion under the temperature ranging from minus 60 DEG C to minus 10 DEG C, adding precooled solidification liquid, carrying out reverse-phase regeneration and thus obtaining the chitosan-based porous microspheres. The preparation method has the beneficial effect that the surfaces and the inner parts of the porous microspheres have porous structures with communicated heights.
Owner:福建梅生药业有限公司
Chitin tetra ammonium salt nano-particle, its preparation method and use
InactiveCN1686560AGood water solubilityGood sustained release effectPowder deliveryPharmaceutical non-active ingredientsFreeze-dryingSolvent
A chitosen-quaternary ammonium salt nanoparticle as the carrier of protein-type medicine is prepared through dissolving chitosan-quaternary ammonium salt in distilled water, stirring while adding solution of sodium tripolyphosphate, cross-linking, centrifugal separation, and freeze drying. It has long relesing period (6 days).
Owner:WUHAN UNIV
Vaccine composition containing synthetic adjuvant
InactiveUS20110014274A1SsRNA viruses negative-senseBacterial antigen ingredientsNatural productDrug carrier
Compositions and methods, including vaccines and pharmaceutical compositions for inducing or enhancing an immune response are disclosed based on the discovery of useful immunological adjuvant properties in a synthetic, glucopyranosyl lipid adjuvant (GLA) that is provided in substantially homogeneous form. Chemically defined, synthetic GLA offers a consistent vaccine component from lot to lot without the fluctuations in contaminants or activity that compromise natural-product adjuvants. Also provided are vaccines and pharmaceutical compositions that include GLA and one or more of an antigen, a Toll-like receptor (TLR) agonist, a co-adjuvant and a carrier such as a pharmaceutical carrier.
Owner:INFECTIOUS DISEASE RES INST
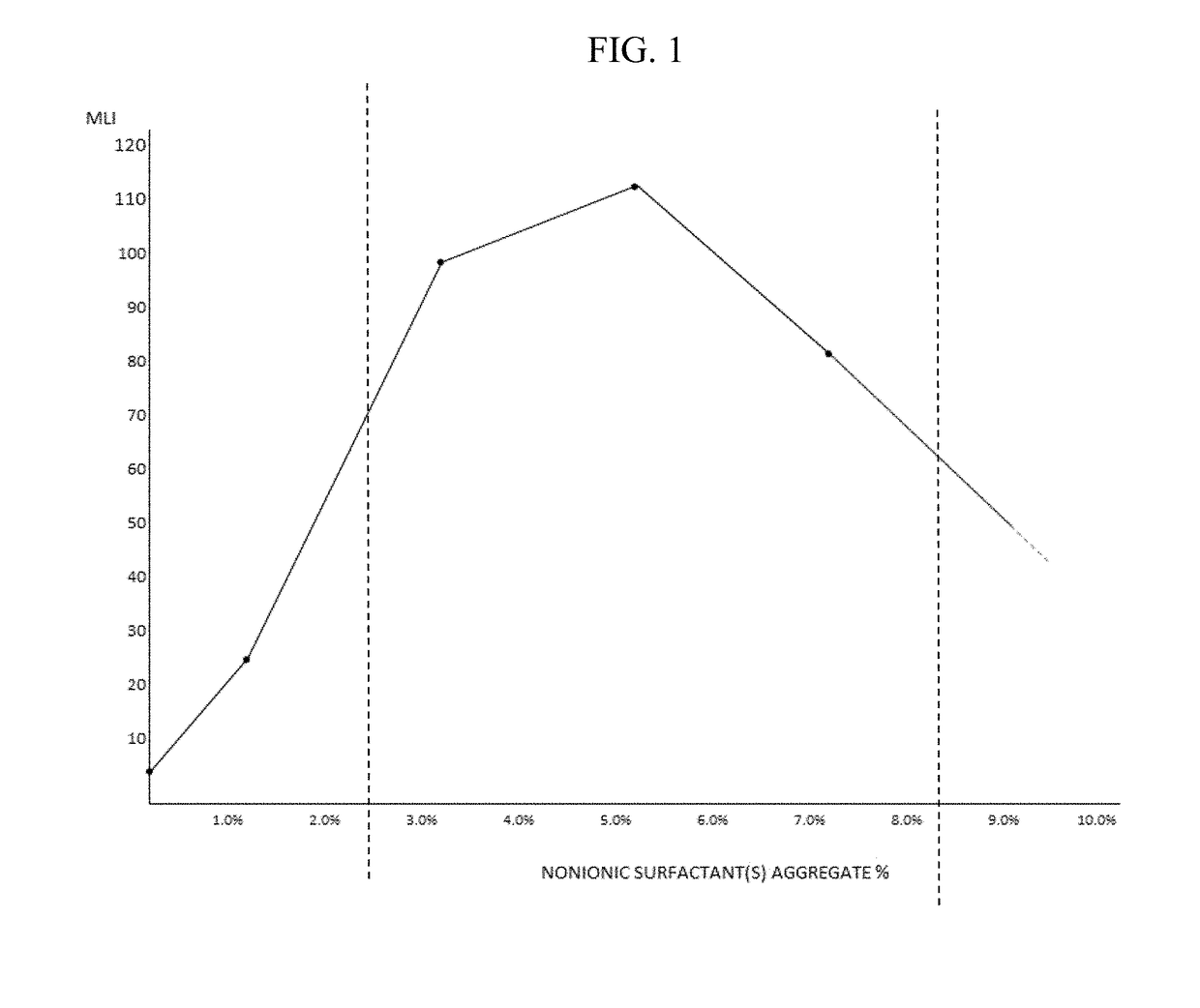
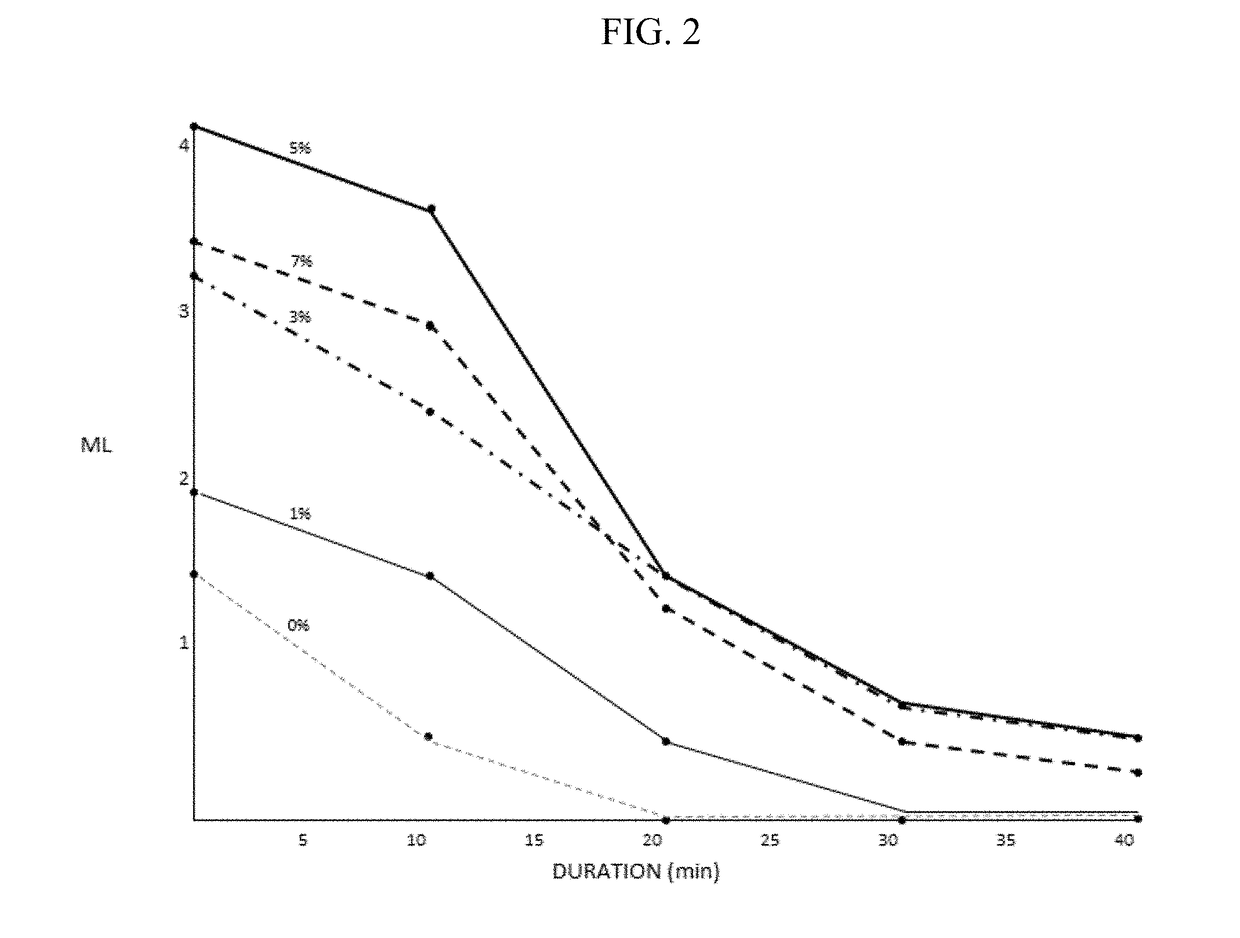
Artificial tear, contact lens and drug vehicle compositions and methods of use thereof
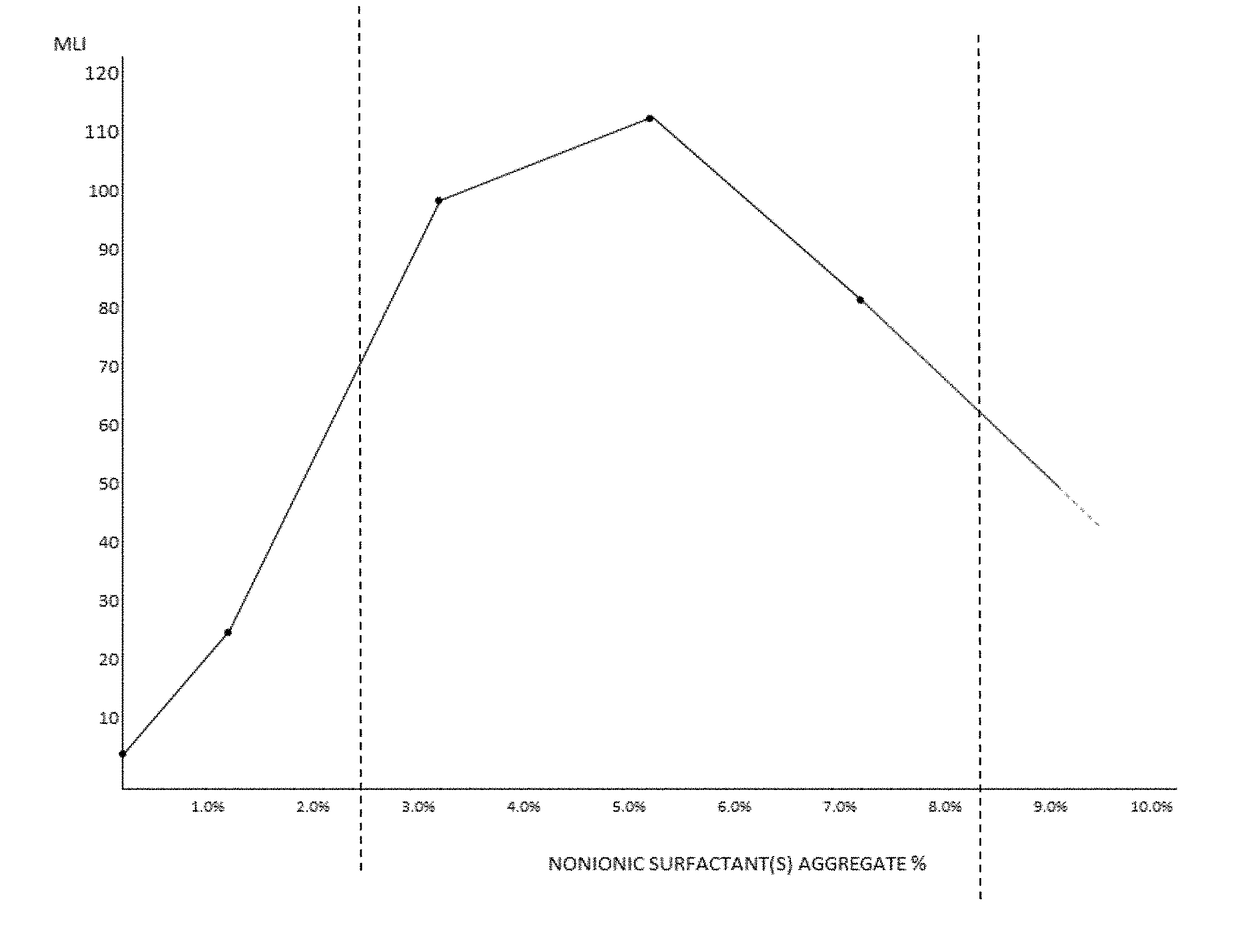
PendingUS20180098937A1Enhancing contact lens wear timeReduce decreaseOrganic active ingredientsSenses disorderPolyolMedicine
The invention provides artificial tear compositions, artificial tear-gel compositions, contact lens storage compositions, contact lens treatment compositions, ophthalmological drug vehicle compositions and topical drug vehicle compositions comprising one or more nonionic surfactants with one or more non-Newtonian viscosity enhancing excipients and one or more of a polyol and or an electrolyte and methods of their use.
Owner:PS THERAPIES LTD +1
Human appetite control by glucagon-like peptide receptor binding compounds
InactiveUS20060128627A1Appetite suppressantReducing spontaneous food intakePeptide/protein ingredientsMetabolism disorderCompound aCompound (substance)
A composition including a compound which binds to a receptor for glucagon-like peptide-1 and a pharmaceutical carrier. The amount of the compound present is effective to control appetite in a human. Also disclosed is a method for controlling appetite and for reducing food intake in a human by administering to the human a composition comprising a compound which binds to a receptor for glucagon-like peptide-1 and a pharmaceutical carrier.
Owner:AMYLIN PHARMA INC
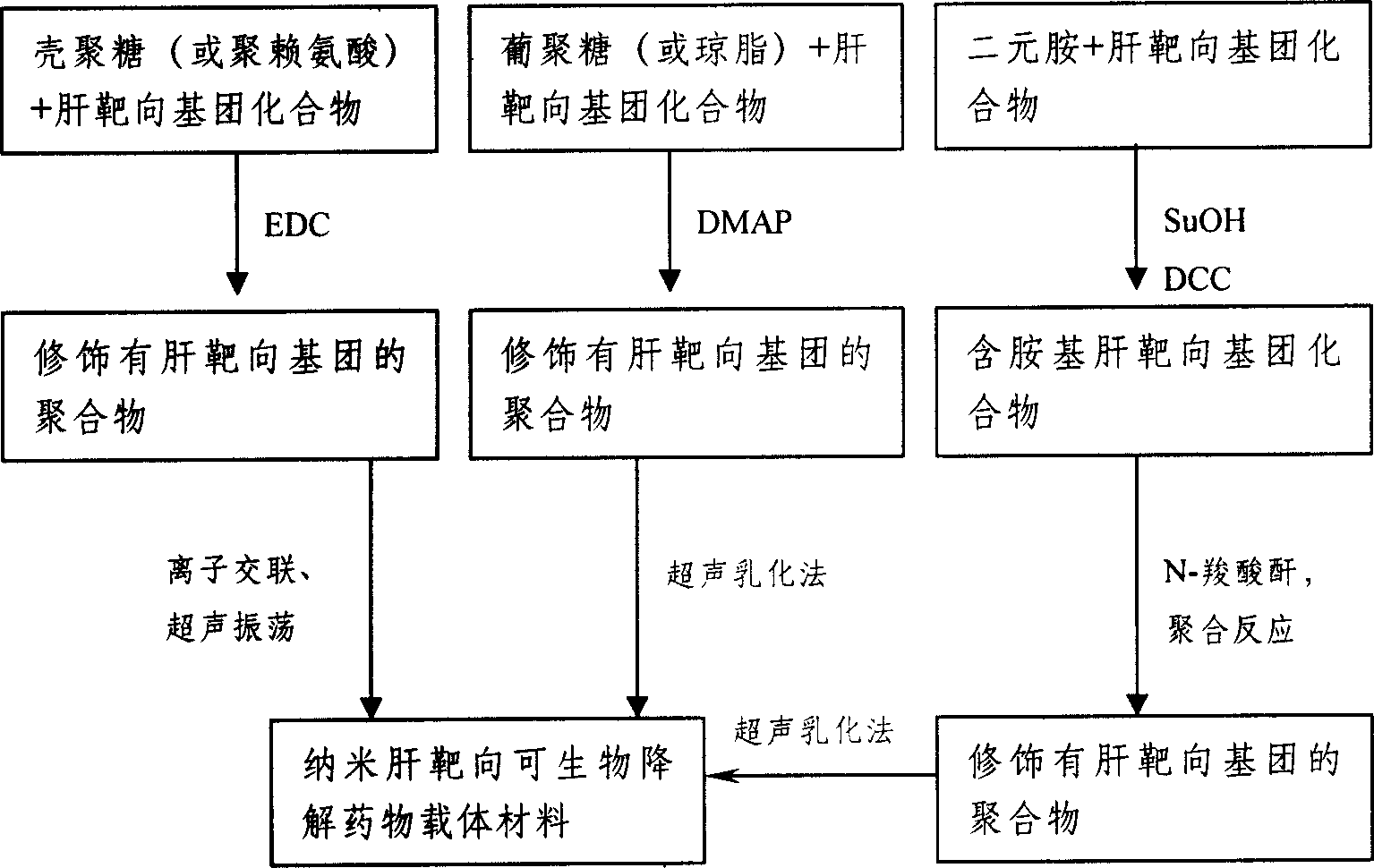
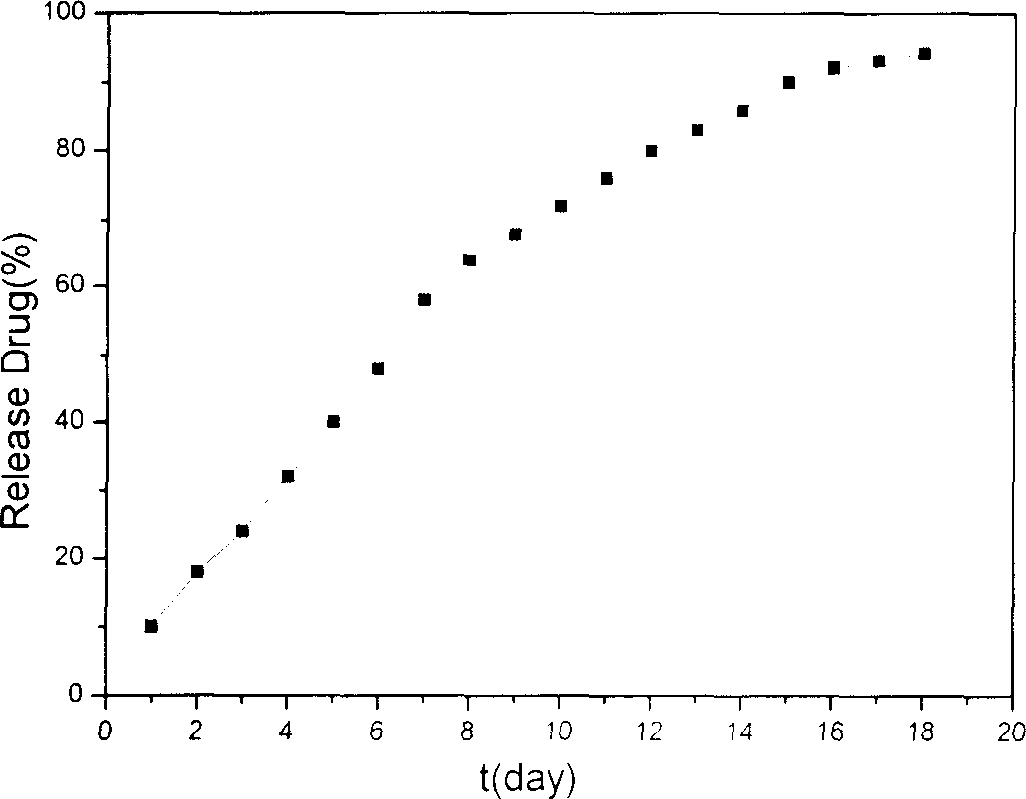
Method for preparing nano liver-target biodegradating medicine carrier material
InactiveCN1743008ATargeted sustained-release therapy achievedNo side effectsOrganic active ingredientsPharmaceutical non-active ingredientsUltrasonic emulsificationIon exchange
The preparation method includes the following steps: modifying hepatic target compound onto degradable polymer (chitosan, polylysine, glucosan, agar, polyglutamic acid-benzyl ester, polyalanine) with biological compatibility, and adopting ion exchange or ultrasonic emulsification process to obtain the invented nano hepatic target bio-degradable medicine carrier material. The hepatic target nano particle solution has good target performance for liver, the medicine enriched rate in the liver can be up to 75%, and its slowly-released administration can be up to above 15 days.
Owner:NANKAI UNIV
Methods and formulations for delivery of pharmacologically active agents
InactiveUS20060073175A1Short timeEliminate side effectsOrganic active ingredientsEchographic/ultrasound-imaging preparationsActive agentSide effect
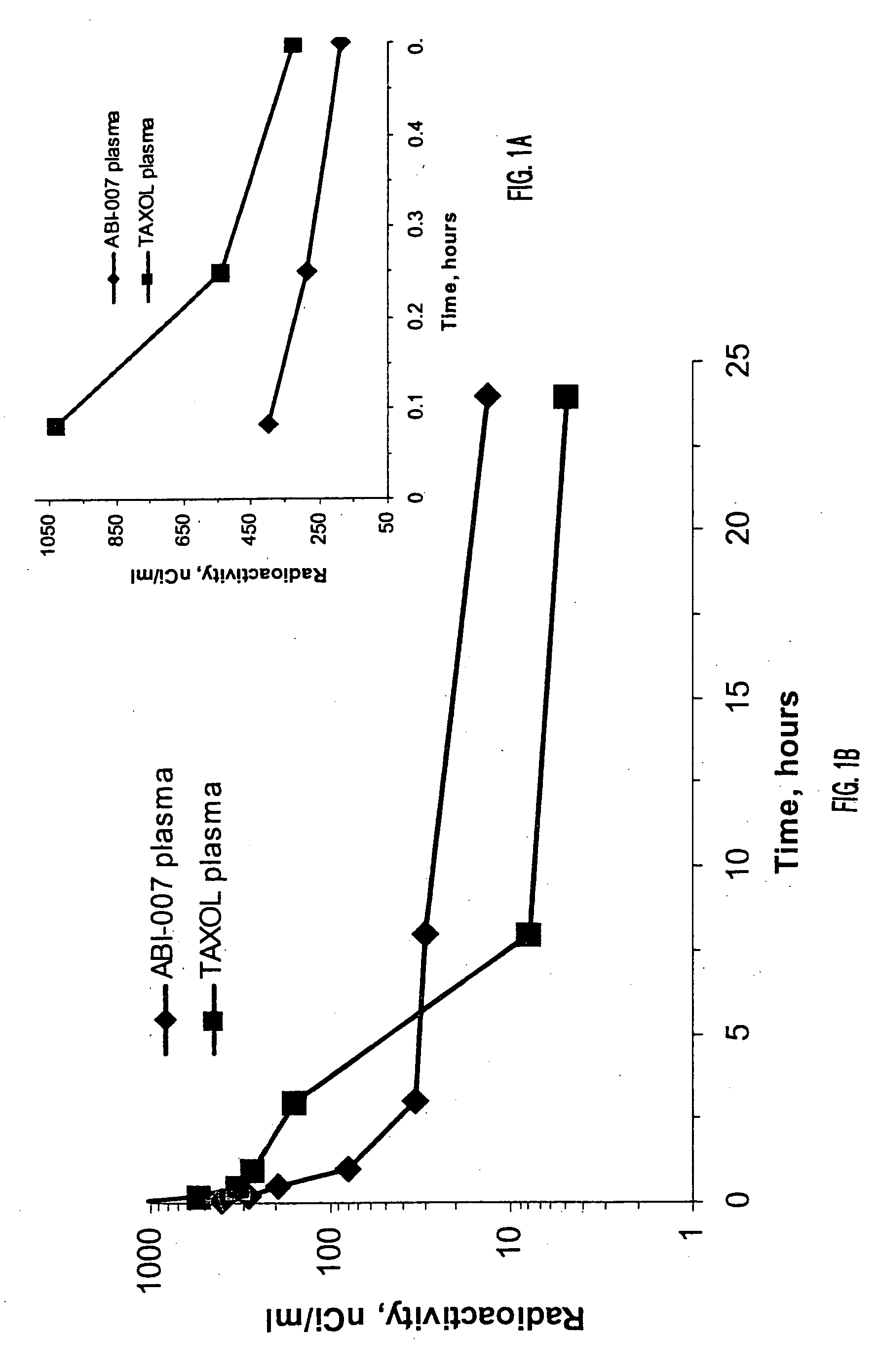
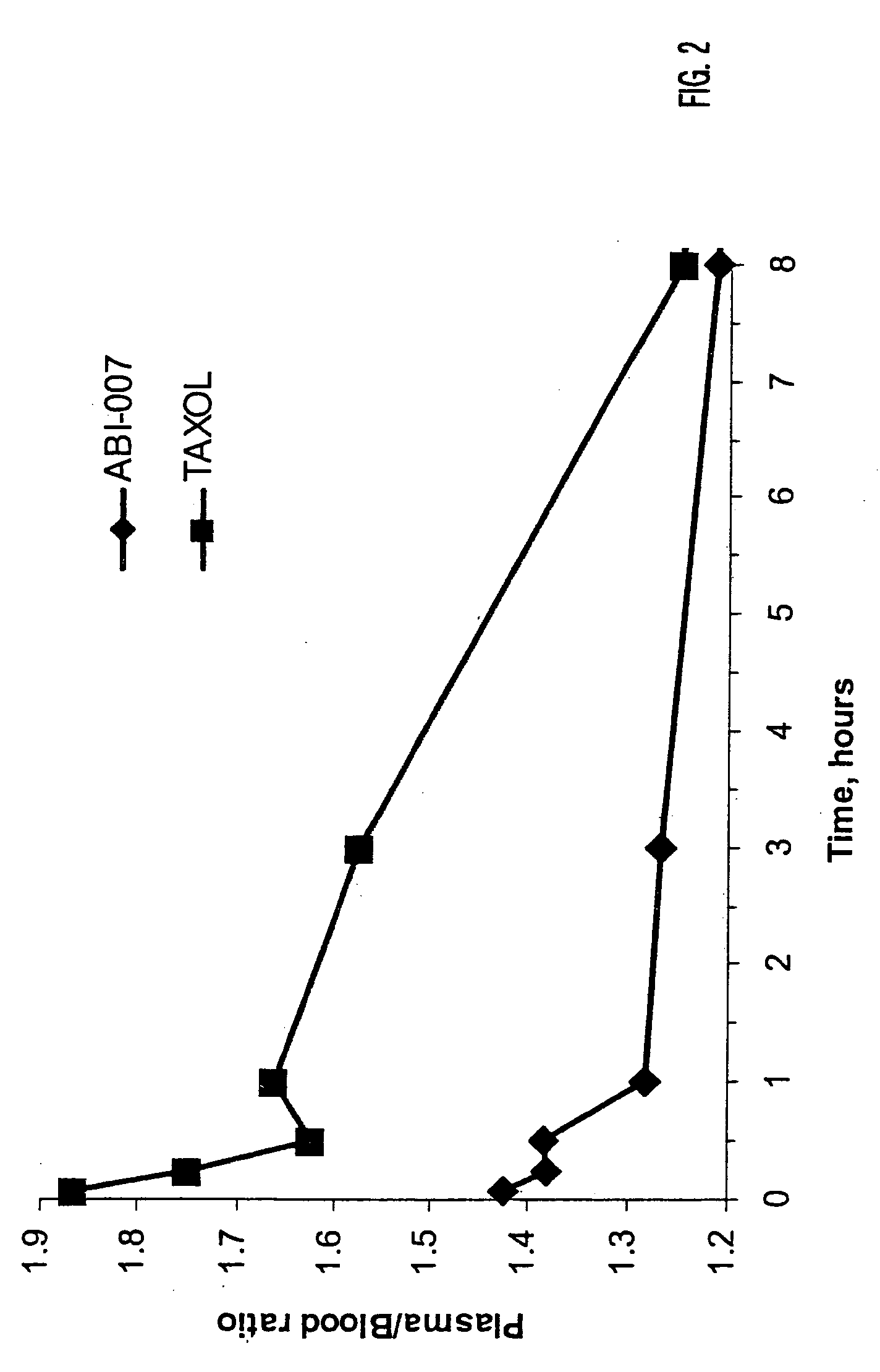
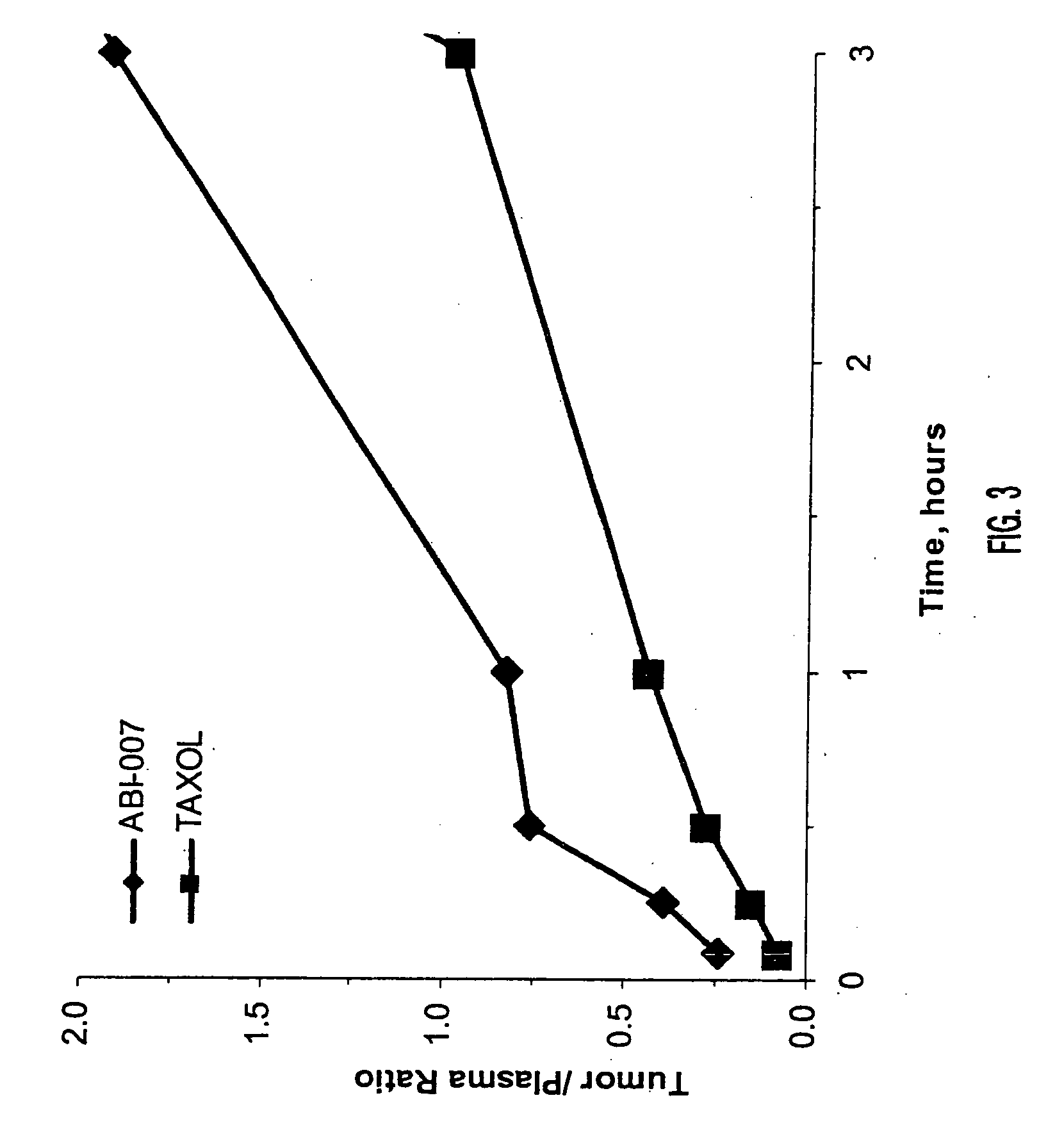
In accordance with the present invention, novel formulations have been developed which are much more effective for the delivery of hydrophobic drugs to patients in need thereof than are prior art formulations. Invention formulations are capable of delivering more drug in shorter periods of time, with reduced side effects caused by the pharmaceutical carrier employed for delivery.
Owner:ABRAXIS BIOSCI LLC
High molecular anticarcinogenic prodrug, its preparing method and use
ActiveCN101045163ALymphatic targetingTargetedOrganic active ingredientsPeptide/protein ingredientsMolecular precursorDrainage lymph nodes
An anticancer high-molecular precursor medicine C-Spacer-P, where P is high- molecular pectin, C is an anticancer medicine containing amino radical or hydroxy radical, and Spacer is the spacing radical, is prepared by bonding the amino or hydroxy of an anticancer medicine with the hydroxy, carboxy, or hydroxymethyl of high- molecular pectin via spacing radical. It is an injection suitable for the solid cancer, and cancerous ascites or hydrothorax.
Owner:SICHUAN YINGRUI PHARMA TECH CO
Vehicle and method for treating and preventing acne vulgaris and exfoliating the skin hypohalous acids
InactiveUS20080014289A1Effective formulationCosmetic preparationsBiocideCleansers skinHypobromous acid
Vehicle and method is disclosed for treating and / or preventing skin disorders such as acne vulgaris and which exfoliates healthy skin by a topical application. The vehicle is a formulation incorporating hypohalous acid in a suitable pharmaceutical compound. The topical application comprises administering and scrubbing a therapeutically effective amount of hypohalous acid in the vehicle. The hypohalous acid may be hypochlorous acid, hypobromous acid, or hypoiodious acid. The suitable pharmaceutical vehicles include water, solutions, cleansers, lotion, cream, paper facial masks, and gels. The scrubbing action on the skin is exerted by a mechanic tool, such as hand, cloth towel, sponge, brush, and spraying device. Methods of preparing and compounding the vehicle for topical application and of its use are also set forth.
Owner:LI JIANPING
Mono-layer oxidized mineral carbon and ferriferrous oxide composite material as well as preparation and application
InactiveCN101474406AHigh efficiency loadGood dispersionInorganic non-active ingredientsSuperparamagnetismNitrogen gas
The invention relates to a monolayer graphite oxide and magnetic ferroferric oxide nano-particle composite hybrid material, and a preparation method and application thereof. The monolayer graphite oxide and magnetic ferroferric oxide nano-particle composite hybrid material is made from raw materials of monolayer graphite oxide material and a mixture of bivalent malysite and ferric malysite by a chemical deposition method. The preparation method comprises the steps as follows: the monolayer graphite oxide is dispersed in sodium hydroxide aqueous solution to obtain monolayer graphite oxide with sodium carboxylate; then ion exchange is carried out on the mixture of monolayer graphite oxide and the bivalent malysite and the ferric malysite under the protection of nitrogen; the processed mixture is deposited by sodium hydroxide solution after excessive malysite is removed to obtain a solid product; and the solid product is separated out and dried to obtain superparamagnetic monolayer graphite oxide and ferroferric oxide nano-particle composite hybrid material. The material is used for loading drug to obtain an efficient controllable targeted drug carrier with the magnetic property of pH response. The invention has wide application prospect in the aspects of multiple targeted drug delivery, and the separation, the purification, the inspection and the like of nuclear magnetic resonance contrast medium, DAN, protein, etc.
Owner:TIANJIN MEDICAL UNIV
Method of mitigating adverse drug events using omega-3-fatty acids as a parenteral therapeutic drug vehicle
A method of parenterally administering a composition, the method including parenterally administering to a person a composition including at least one omega-3 fatty acid and at least one drug, wherein the at least one omega-3 fatty acid source and the at least one drug are administered simultaneously.
Owner:STABLE SOLUTIONS
Coronary artery skeleton medicinal coating for preventing restenosis of blood vessel
InactiveCN1429547AAchieve constant rate releaseOrganic active ingredientsSurgeryEpoxyEthylene Oxide Sterilization
A coated medicine layer on the scaffold in arteria coronaria for preventing angiostenosis is prepared through dissolving the aliphatic polylactone and its copolymer is solvent, adding taxusol, stirring while dissolving, filtering, coating on the scaffold, evaporating the solvent, removing the solvent for 48 hr under vacuum, and disinfecting by epoxy ethane.
Owner:INST OF CHEM CHINESE ACAD OF SCI +1
Fatty acylaminoacylcytarabine conjugate, preparation method and application thereof
InactiveCN101240002AStrong half lifeStrong bioavailabilityOrganic active ingredientsSugar derivativesCytarabineMedicine
The invention discloses a conjugate of fatty aminoacyl alexan, preparation and application in anti-tumor thereof. The invention also discloses a pharmacosome of the conjugate of fatty aminoacyl alexan, preparation thereof, and its applicant in anti-tumor and preparation of target medicine material of microemulsion, lipid medicine carrier. In comparison with alexan, the inventive conjugate of fatty aminoacyl alexan has a strong transmembrane ability, high bioavailability, long halflife, and amphipathic nature. It is demonstrated that the inventive compound and pharmacosome have great anti-tumor activity.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
Water in oil formulations, method to prepare same, and personal care products formed using same
A stable suspension formed of a plurality of water particles each of which is encapsulated with an amorphous silica-based material, where that plurality of encapsulated water particles is dispersed in a continuous phase formed of one or more carbon-containing materials, where the stable suspension does not include added emulsifiers. A method to prepare such stable suspensions. Personal care products comprising Applicant's stable suspensions, including skin protectants, sunscreens, moisturizers, vehicles for medicaments, antiperspirants, deodorants, pressurized products such as aerosol products, vehicles for skin treatment products and vehicles for makeup, area of the eye, lip products, mascara and color cosmetic products.
Owner:DESERT WHALE JOJOBA
Methods and compostions for enhancing transdermal drug delivery
Owner:NAT INST OF HEALTH REPRESENTED BY THE SEC OF THE DEPT OF HEALTH & HUMAN SERVICES NAT INST OF HEALTH
Hair growth formula
A hair treatment composition for topical application to the skin and / or hair, comprising partially hydrolyzed fucoidan and a pharmaceutical carrier. The composition may contain further additives. The carrier may be hydrophyllic or anhydrous. The composition may also include natural components such as honey and / or mangosteen. The composition may also include flavonoids, analgesics, radiation protecting agents, and / or anti-oxidants.
Owner:SAKURA PROPERTIES LLC
Dicarboxylic acid foamable vehicle and pharmaceutical compositions thereof
Owner:VYNE THERAPEUTICS INC
Anti-pathogenic composition useful in blood preservation
InactiveUS20050053516A1Safe and effective useDecrease in levelAntibacterial agentsBiocideProtozoaAmmonium compounds
The present invention includes a method for reducing viral, bacterial, protozoan, fungal and other parasitic contamination from a biological solution. Biological solutions include, but are not limited to, solutions comprising blood, a blood component, cell culture or a component of a cell culture. In accordance with the present invention, there is provided, a method of inactivating a pathogen in blood or blood product in a container, comprising contacting at least one of said blood, blood product and container with a composition of the present invention. In accordance with the present invention, there is provided, a medical device comprising at least a surface treated with an anti-pathogenic composition of the present invention or containing at least an anti-pathogenic composition of present invention. In accordance with the present invention, there is provided, an anti-pathogenic composition for use in disinfecting fluids and biological tissues and surfaces contaminated with fluids and / or biological tissues, which comprises an anti-pathogenic amount of at least one quaternary ammonium compound in association with an acceptable carrier. A preferred anti-pathogenic composition of the present invention further comprises a bisguanidine compound. In accordance with the present invention, there is provided a method for inhibiting in vitro or ex vivo infection or replication of human immunodeficiency virus in a biological fluid, comprising treating said biological fluid with an effective inhibiting amount of a bis-guanidine compound or a derivative thereof, and at least one quaternary ammonium compound in combination with a pharmaceutically carrier, such as DMSO.
Owner:ALTACHEM PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com