Fatty acylaminoacylcytarabine conjugate, preparation method and application thereof
A technology of fatty amidoacyl cytarabine and fatty amido arabinocytidine, which is applied in sugar derivatives, drug combinations, pharmaceutical formulations, etc., and can solve problems such as low bioavailability, weak transmembrane ability, and short half-life. problem to achieve good antitumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
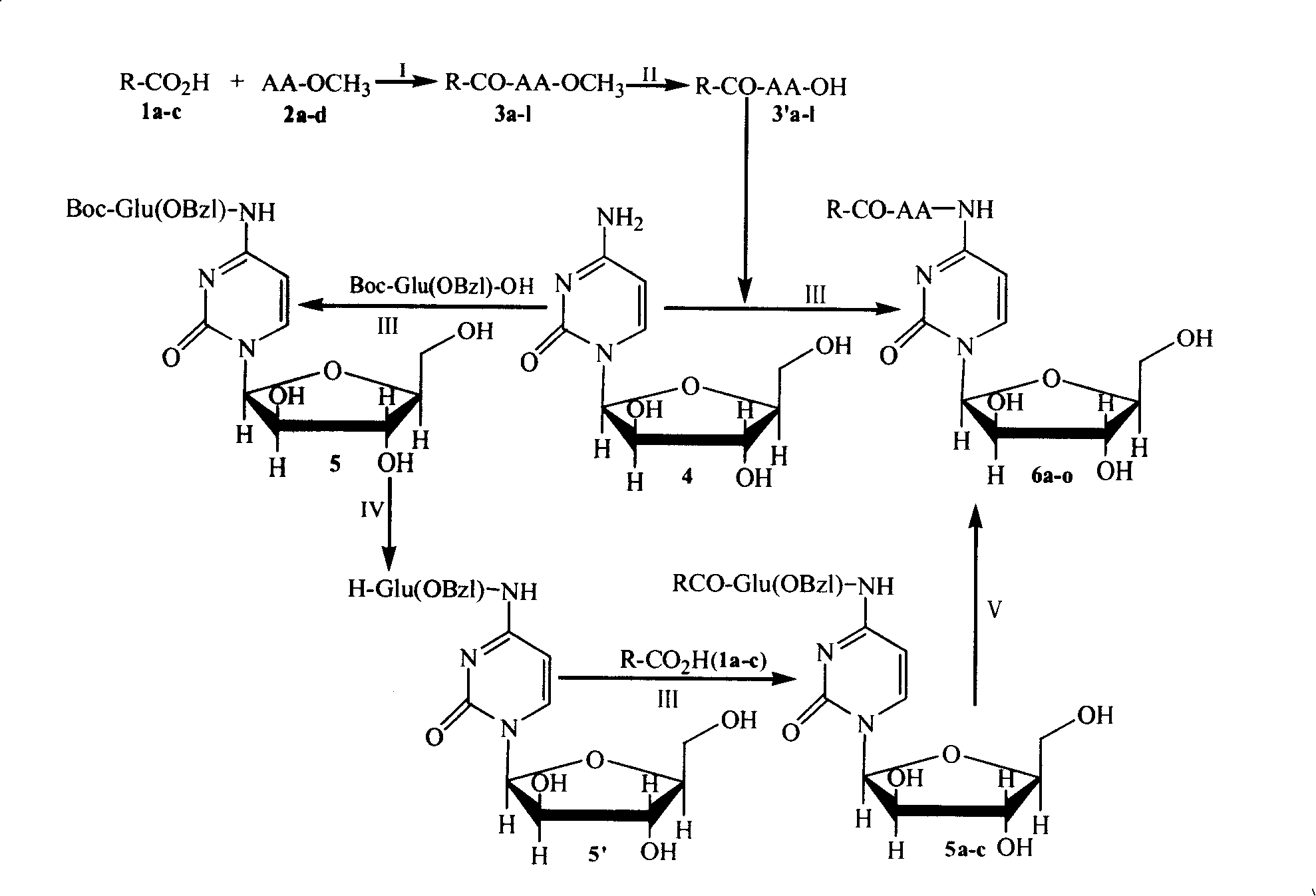
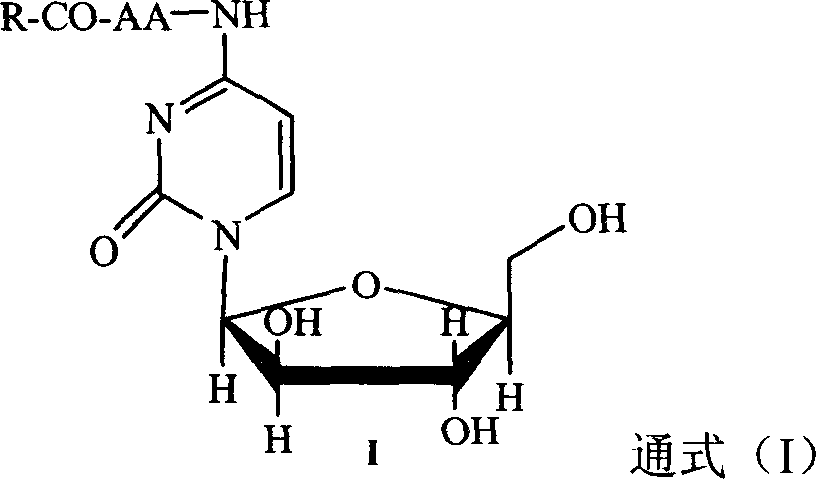
Image
Examples
Embodiment 1
[0024] Embodiment 1 prepares caproyl valine methyl ester (3a)
[0025] Dissolve 0.69g (4mmol) capric acid, 0.91g (4.4mmol) DCC and 0.54g (4mmol) HOBt in cold anhydrous THF, stir in ice bath for 20min, then add 0.67g (4mmol) HCl·H-Val-OMe Adjust the pH value to 8-9 with 0.5ml NMM, stir at room temperature for 1 day, TLC (chloroform / methanol, 20:1) showed that HCl·H-Val-OMe disappeared. Dicyclohexyl urea (DCU) was filtered off, the filtrate was concentrated to dryness under reduced pressure, and the residue was dissolved in 20 ml of ethyl acetate. The resulting solution was sequentially washed with 5% NaHCO 3 Washing with aqueous solution, washing with saturated NaCl aqueous solution, 5% KHSO 4 Wash with aqueous solution and saturated NaCl aqueous solution until neutral. Ethyl acetate layer with anhydrous Na 2 SO 4 Dry, filter, and concentrate the filtrate under reduced pressure to afford 1.01 g (89%) of the title compound as a colorless solid. Mp.60-62℃; [α] D 25 20 =-...
Embodiment 2
[0026] Embodiment 2 prepares caproyl methionine methyl ester (3b)
[0027] According to the operation of Example 1, 1.17g was prepared from 0.69g (4mmol) capric acid, 0.91g (4.4mmol) DCC, 0.54g (4mmol) HOBt, 0.8g (4mmol) HCl H-Met-OMe and 0.5mlNMM (92%) The title compound as a colorless solid. Mp.50-52℃; [α] D 25 20 =-19.5 (c=1.0, methanol); ESI-MS (m / e) 318.0 [M+H] + , 635.1[2M+H] + , 657.1[2M+Na] + .
Embodiment 3
[0028] Embodiment 3 prepares caproyl tyrosine methyl ester (3c)
[0029] According to the operation of Example 1, 1.09g was prepared from 0.69g (4mmol) capric acid, 0.91g (4.4mmol) DCC, 0.54g (4mmol) HOBt, 0.93g (4mmol) HCl H-Tyr-OMe and 0.5mlNMM (80%) The title compound as a colorless solid. Mp.27-29℃; [α] D 25 20 =-17.7 (c=1.0, methanol); ESI-MS (m / e) 350.0 [M+H] + , 699.2[2M+H] + .
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com