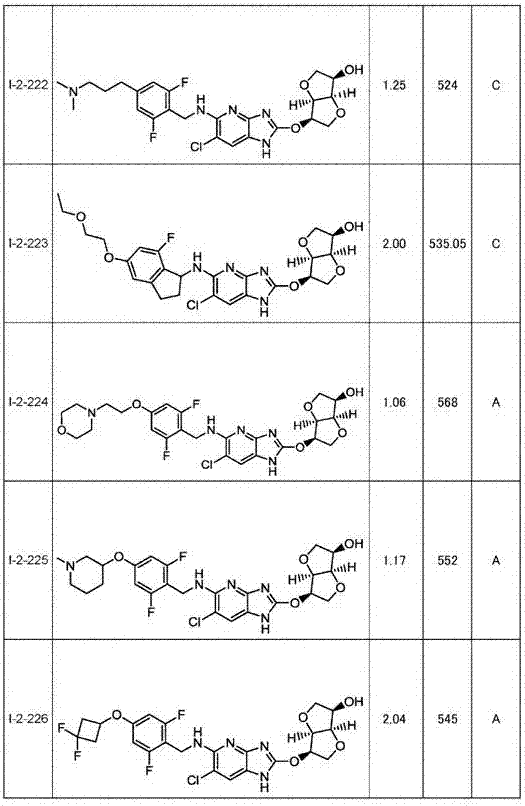
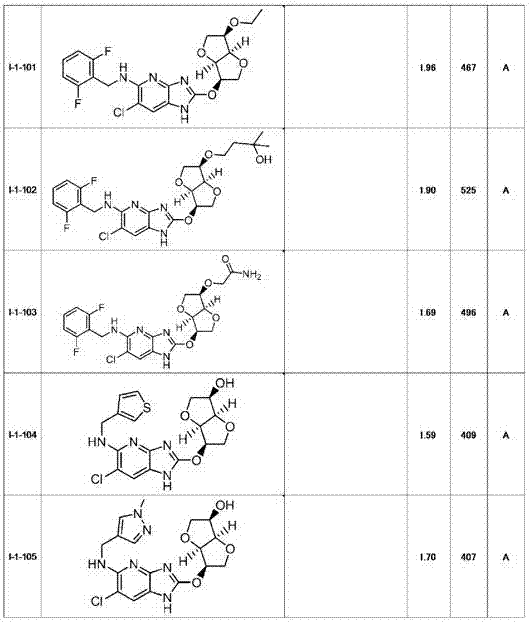
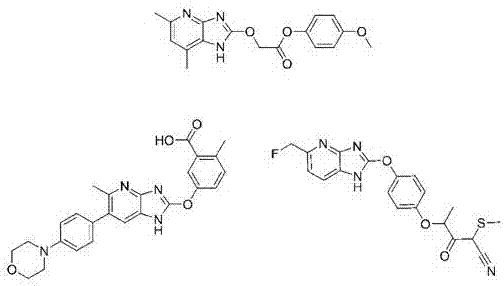
Heterocyclic derivative having ampk activating effect
A technology of compounds and heterocyclic groups, applied in the field of heterocyclic derivatives with AMPK activation, can solve the problems of unclear activation of AMPK and no literature information.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0698] [chemical formula 40]
[0699]
[0700] At room temperature, Ruphos (40.5 mg, 0.087 mmol), Pd 2 (dba) 3 (39.7mg, 0.043mmol) and NaOt-Bu (167mg, 1.73mmol) were stirred under microwave irradiation at 120°C for 10 minutes. The reaction solution was purified by silica gel column chromatography, whereby Compound 3 (274 mg, 0.400 mmol, 46%) was obtained as a colorless liquid.
[0701] Compound 3; retention time = 3.30 min, mass (M+H) = 683.35, method = C.
[0702] A THF solution of TBAF (1M solution, 6.00 mL, 6.00 mmol) was added to Compound 3 (274 mg, 0.400 mmol), and stirred at 80° C. for 6 hours. The reaction solution was purified by silica gel column chromatography and solidified with hexane / ethyl acetate to obtain Compound I-1-1 (81.4 mg, 0.186 mmol, 46%) as a white solid.
[0703]
Embodiment 2
[0705] [chemical formula 41]
[0706]
[0707]
[0708] At room temperature, Ruphos (40.5mg, 0.087mmol), Pd 2 (dba) 3 (39.7mg, 0.043mmol) and NaOt-Bu (167mg, 1.73mmol) were stirred under microwave irradiation at 120°C for 10 minutes. The reaction solution was purified by silica gel column chromatography, whereby Compound 5 (274 mg, 0.404 mmol, 47%) was obtained as a yellow liquid.
[0709] Compound 5; retention time = 3.26 min, mass (M+H) = 677.45, method = C.
[0710] Trifluoroacetic acid (1.2 mL, 15.8 mmol) was added to a solution of compound 5 (245 mg, 0.362 mmol) in dichloromethane (1.2 mL), followed by stirring at 0° C. for 1 hour. Under ice-cooling, the reaction solution was quenched with saturated aqueous sodium bicarbonate solution, extracted with ethyl acetate, and the solvent was distilled off under reduced pressure to obtain a crude product (196 mg) containing compound 6, which was directly used in the next reaction.
[0711]Add pyridine (0.069mL, 0.857mm...
Embodiment 3
[0715] [chemical formula 42]
[0716]
[0717] To compound 1 (50mg, 0.087mmol), Pd(OAc) 2 (1.947mg, 0.00867mmol), BINAP (5.40mg, 0.00867mmol), K 2 CO 3 (41.9mg, 0.303mmol), and aniline (9.50 mu L, 0.104 mmol) was added toluene (1 mL), and stirred at 150° C. for 30 minutes under microwave irradiation. Water was added to the reaction liquid, followed by extraction with ethyl acetate, and the solvent was distilled off under reduced pressure. The concentrated residue was purified by silica gel chromatography, whereby Compound 8 (36 mg, 0.057 mmol, 66%) was obtained as a colorless liquid.
[0718]
[0719] Retention time = 3.40 min, mass (M+H) = 633.15, method = C.
[0720] To compound 8 (36mg, 0.057mmol) in dichloromethane (360 mu L) Add TFA (360 mu L), stirred at room temperature for 18 hours. After confirming the disappearance of the raw materials, THF and 2 mol / L sodium hydroxide aqueous solution were added, and the mixture was stirred at room temperature for 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com