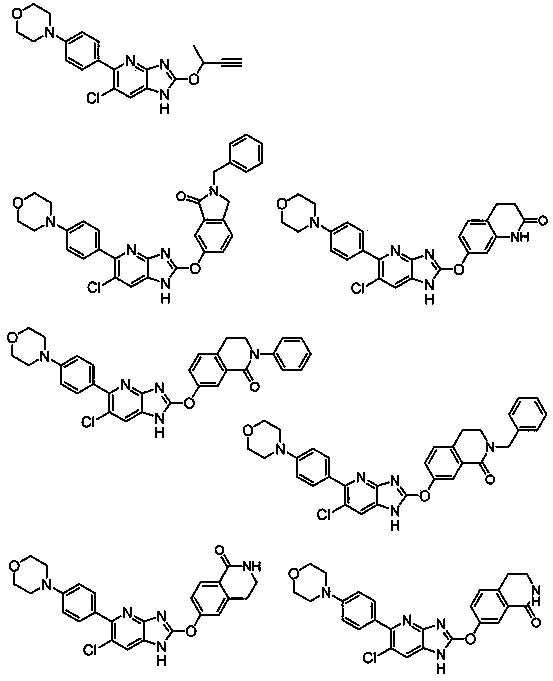
Azabenzimidazole derivative having AMPK-activating activity
A compound, chemical formula technology, applied in the direction of organic chemistry, organic active ingredients, medical preparations containing active ingredients, etc., can solve the problems of AMPK activation is not documented, azabenzimidazole derivatives are not disclosed, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0479] [chemical formula 17]
[0480]
[0481] While stirring a suspension of Compound 1 (20 g, 115 mmol) in absolute ethanol (970 mL), chlorine gas was bubbled into it at 0° C. for 1 hour. Then, stirring the reaction liquid, after bubbling nitrogen gas there over 1 hour at room temperature, it stirred at 0 degreeC for 30 minutes. The reaction suspension was collected by filtration and washed with diisopropyl ether, whereby a solid was obtained. The solvent in the obtained filtrate was distilled off under reduced pressure, and the precipitated solid was collected by filtration and washed with diisopropyl ether to obtain a further solid. The above two filtered solids were combined to obtain Compound 2 (18.1 g, 76%) as a yellow solid.
[0482] Compound 2; 1 H-NMR (DMSO-d 6 ) δ: 8.33 (brs, 2H), 8.59 (s, 1H).
[0483] Iron (48.6g, 870mmol) and ammonium chloride (46.5g, 870mmol) were added to compound 2 (36.2g, 174mmol) in ethanol (775mL), water (310mL) solution, stirred at...
Embodiment 2
[0494] [chemical formula 18]
[0495]
[0496]To a suspension of sodium hydride (18.5mg, 0.46mmol) in N,N-dimethylformamide (2mL) was added compound 9 (66.2mg, 0.46mmol) in N,N-dimethylformamide at 0°C Amide (1 mL) solution was stirred at room temperature for 30 minutes. Then, compound 8 (120 mg, 0.21 mmol) was added to the reaction suspension at 0°C, and stirred at room temperature for 1 hour. Further, compound 9 (66.2mg, 0.46mmol) and sodium hydride (18.5mg, 0.46mmol) were sequentially added to the reaction suspension at 0°C, and stirred at room temperature for 30 minutes. Saturated ammonium chloride aqueous solution and ethyl acetate were added to the reaction liquid for extraction, and the organic layer was washed with water and saturated brine, and dried over magnesium sulfate. The solvent was distilled off under reduced pressure, and the residue was purified by silica gel column chromatography to obtain Compound 10 (91.9 mg, 0.16 mmol, 75%) as a colorless amorphous ...
Embodiment 3
[0501] [chemical formula 19]
[0502]
[0503] Sodium hydride (219 mg, 5.47 mmol) was slowly added to a solution of compound 11 (1 g, 4.97 mmol) in DMF (10 mL), and stirred under ice-cooling for 45 minutes. Methyl 2-bromoacrylate was added dropwise thereto, and the temperature was raised to room temperature, followed by stirring overnight. Water was added to the reaction solution, followed by extraction with ethyl acetate, the organic layer was washed twice with water, dried over magnesium sulfate, and the solvent was distilled off under reduced pressure. The concentrated residue was purified by silica gel column chromatography (hexane / ethyl acetate=1:1 to 1:4) to obtain Compound 12 (1.03 g, 72%) as a yellow solid.
[0504] Compound 12; 1 H-NMR (CDCl 3 ) δ: 1.62 (3H, d, J = 7.60 Hz), 3.75 (3H, s), 4.99 (2H, s), 5.54 (1H, q, J = 7.44 Hz), 5.99 (1H, d, J = 2.53 Hz), 6.04 (1H, dd, J = 7.60, 2.53 Hz), 7.19 (1H, d, J = 7.60 Hz), 7.35-7.40 (5H, m).
[0505] Pd / C (50 mg) was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com