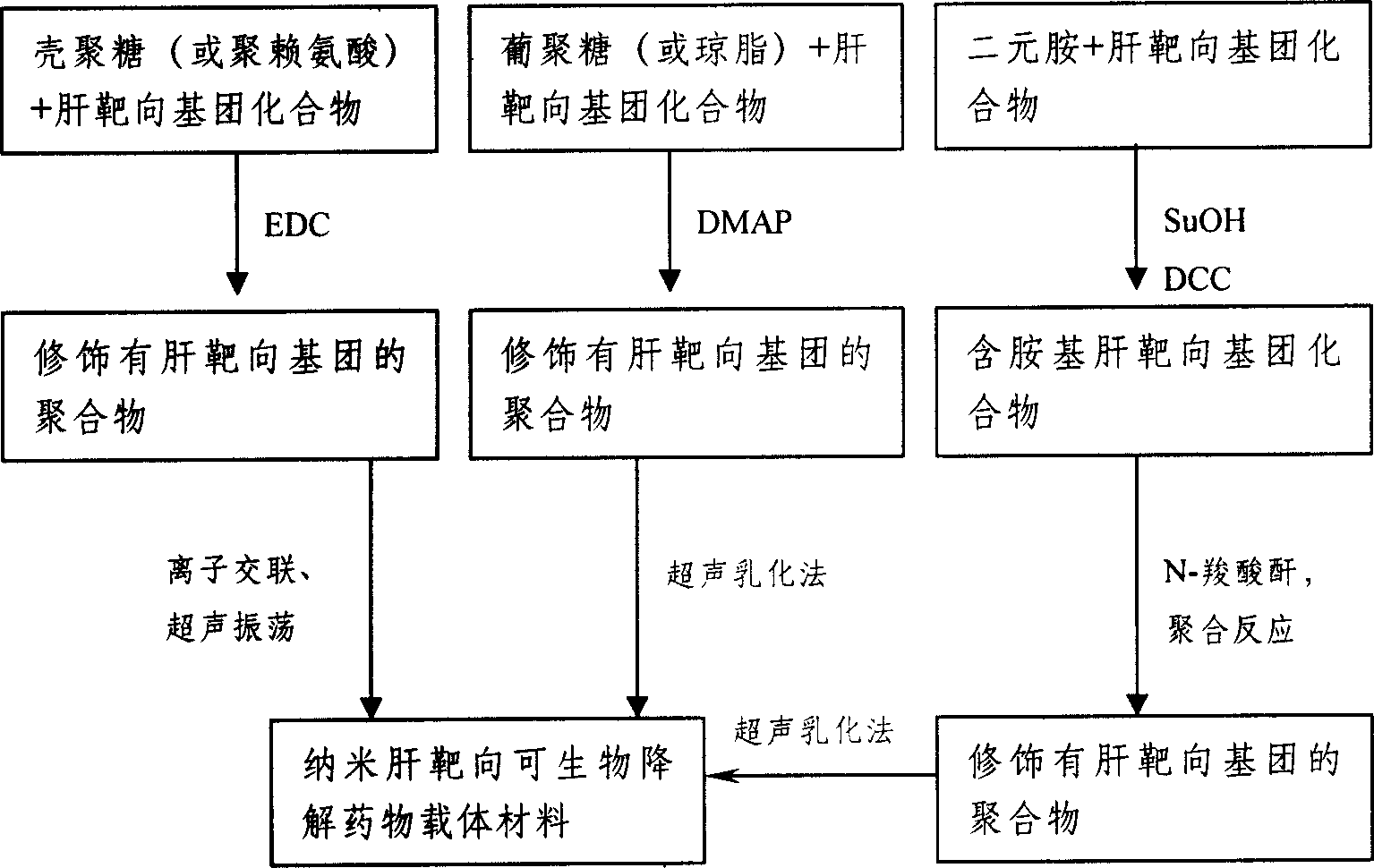
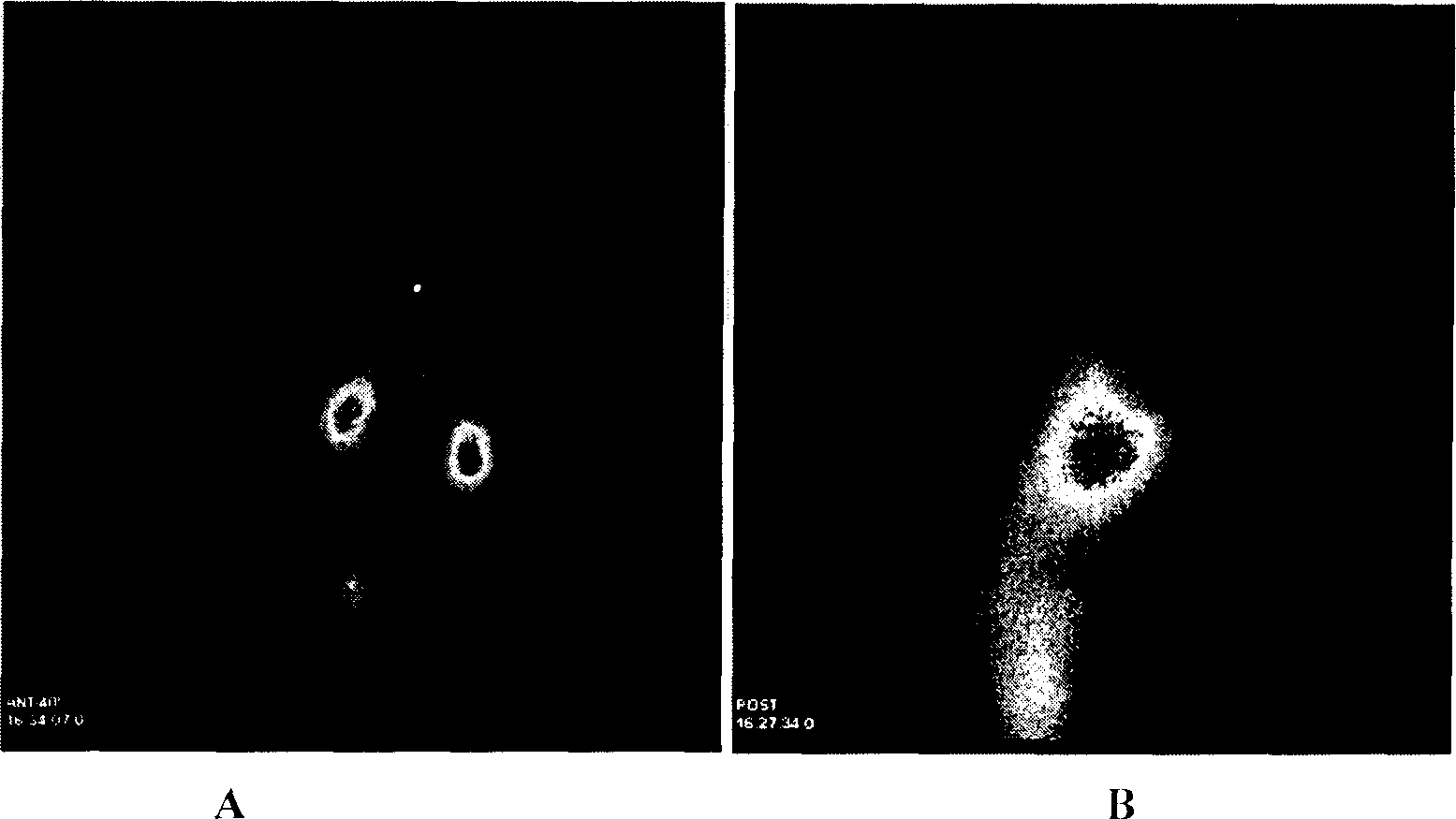
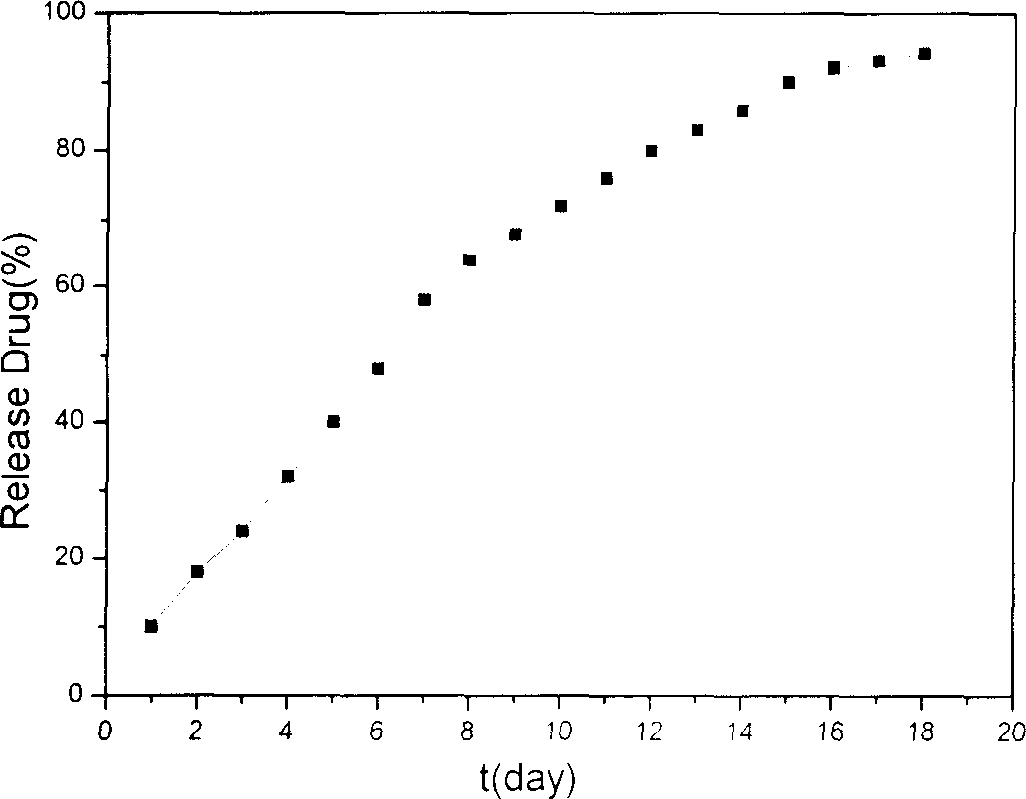
Method for preparing nano liver-target biodegradating medicine carrier material
A carrier material and biodegradable technology, applied in the field of biomedical materials, to achieve good blood compatibility, good targeting ability, and improve the effect of treatment and quality of life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1 Preparation of Glycyrrhetinic Acid-Modified Chitosan Nano Liver Targeted Biodegradation Drug Carrier Material:
[0051] 1.1 Preparation of Glycyrrhetinic Acid Modified Chitosan:
[0052] Weigh 2g of glycyrrhetinic acid and 1g of EDC into a 200mL beaker, add 70mL of DMF into the bottle, and magnetically stir to dissolve.
[0053] Add 5g of chitosan into a 500mL three-neck flask, add 100mL of distilled water and magnetically stir until dissolved. Then pour the glycyrrhetinic acid solution prepared above into the chitosan solution, continue stirring, and heat to 60° C. for 6 hours of reaction. The reaction solution was cooled to 40°C and concentrated to about 50 mL with a rotary evaporator. Pour the concentrated solution into a beaker filled with 800mL ethanol to precipitate, filter, wash the precipitate with 50mL ethanol and 50mL ether respectively, and vacuum dry (40°C, 5mmHg) for 24 hours. The product contains 5% glycyrrhetinic acid.
[0054] 1.2 Preparatio...
Embodiment 2
[0060] Example 2 Preparation of polylysine nano liver targeted biodegradation drug carrier material modified with glycyrrhetinic acid:
[0061] 2.1 Preparation of glycyrrhetinic acid modified polylysine:
[0062] Weigh 2g of glycyrrhetinic acid and 1g of EDC into a 200mL beaker, add 70mL of DMF into the bottle, and magnetically stir to dissolve.
[0063] Add 5g polylysine into a 500mL three-neck flask, add 100mL distilled water and magnetically stir until dissolved. Then pour the glycyrrhetinic acid solution prepared above into the polylysine solution, continue stirring, and heat to 60° C. for 6 hours. The reaction solution was cooled to 40°C and concentrated to about 50 mL with a rotary evaporator. Pour the concentrated solution into a beaker filled with 800mL ethanol to precipitate, filter, wash the precipitate with 50mL ethanol and 50mL ether respectively, and vacuum dry (40°C, 5mmHg) for 24 hours. The product contains 8% glycyrrhetinic acid.
[0064] 2.2 Preparation of ...
Embodiment 3
[0070] Example 3 Preparation of agar nano liver targeted biodegradation drug carrier material modified with glycyrrhetinic acid:
[0071] 3.1 Preparation of Glycyrrhetinic Acid Modified Agar:
[0072] Weigh 2g of glycyrrhetinic acid and 1g of EDC into a 200mL beaker, add 70mL of DMF into the bottle, and magnetically stir to dissolve.
[0073] Add 7g of agar into a 500mL three-neck flask, add 100mL of DMF and magnetically stir until dissolved. Then pour the glycyrrhetinic acid solution prepared above into the agar solution, continue to stir, and heat to 60° C. for 6 hours to react. The reaction solution was cooled to 40°C and concentrated to about 50 mL with a rotary evaporator. Pour the concentrated solution into a beaker filled with 800mL ethanol to precipitate, filter, wash the precipitate with 50mL ethanol and 50mL ether respectively, and vacuum dry (40°C, 5mmHg) for 24 hours. The product contains 4% glycyrrhetinic acid.
[0074] 3.2 Preparation of Glycyrrhetinic Acid Mo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com