Impurity in ziprasidone hydrochloride and preparation method thereof
A technology of ziprasidone hydrochloride and ziprasidone, which is applied in the field of ziprasidone hydrochloride impurity research, can solve problems such as the difficulty of impurity reference substances, and achieve the effects of reducing drug risk, simple method, and strong controllability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
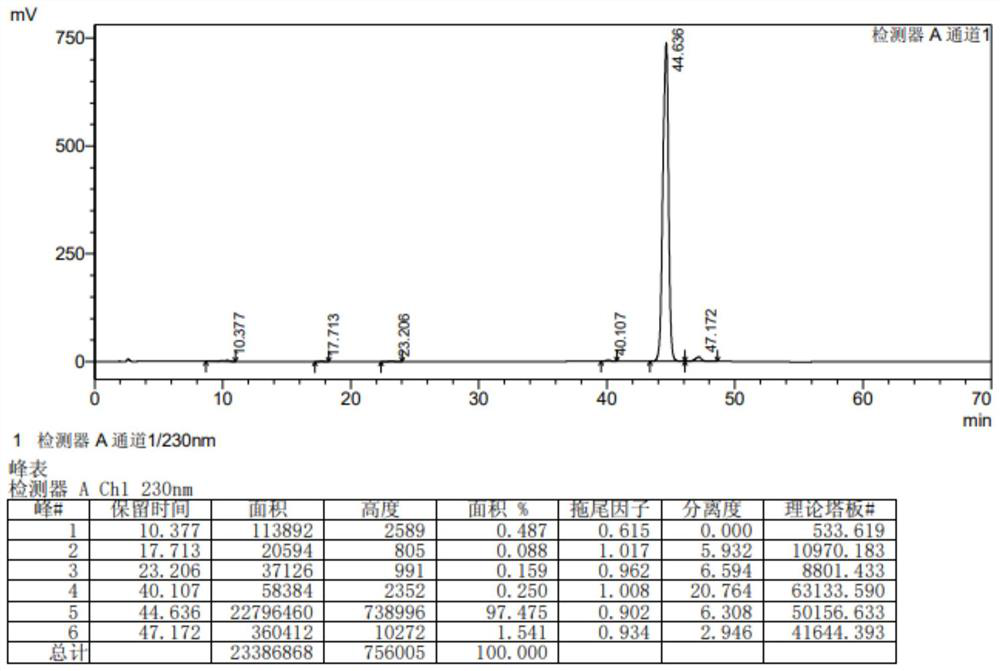
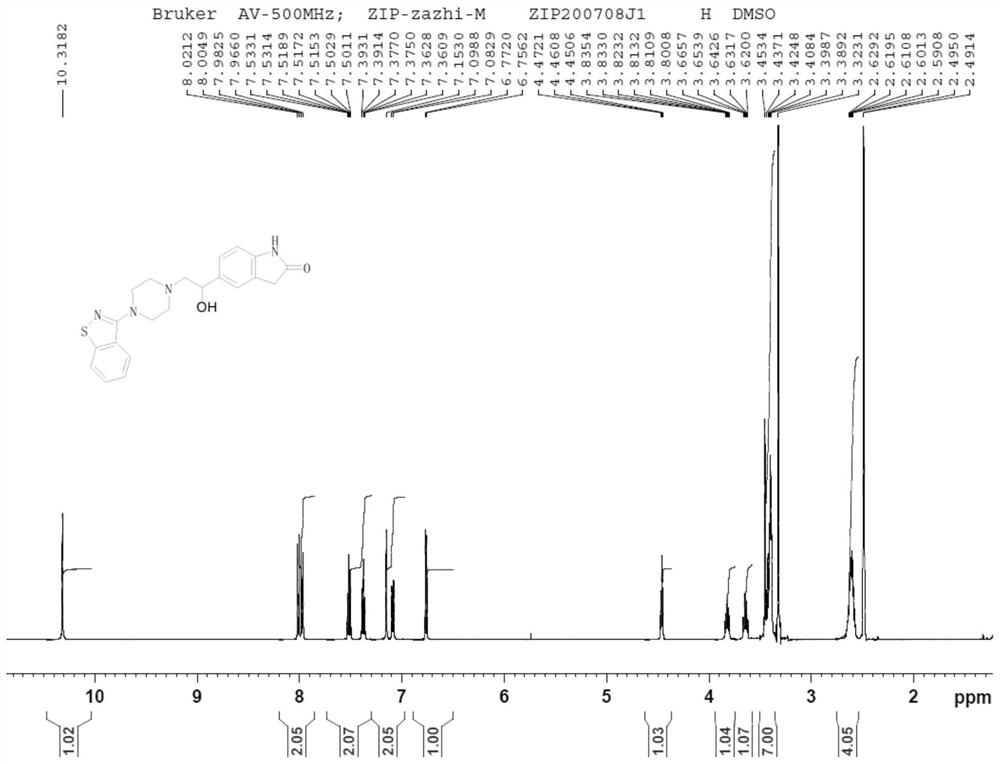
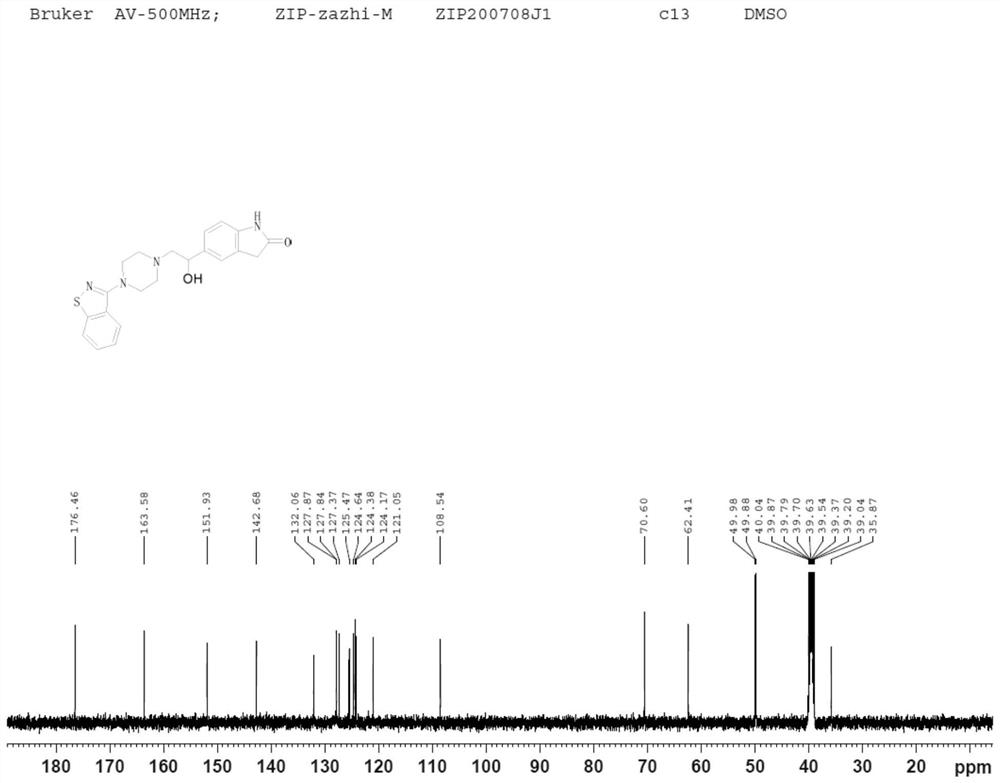
Image
Examples
preparation example Construction
[0070] The invention provides a kind of preparation method of the impurity in ziprasidone hydrochloride, comprises the following steps:
[0071] 1) After mixing the Lewis acid and the first solvent, under a protective atmosphere, after adding chloroacetyl chloride or bromoacetyl bromide, and then adding 1,3-dihydro-indol-2-one for Friedel-Crafts acylation reaction , to give 5-(2-chloro-acetyl)-1,3-dihydro-indol-2-one or 5-(2-bromo-acetyl)-1,3-dihydro-indol-2- ketone;
[0072] 2) The 5-(2-chloro-acetyl)-1,3-dihydro-indol-2-one or 5-(2-bromo-acetyl)-1,3-dihydro- After indol-2-one, reducing agent and second solvent carry out reduction reaction, obtain 5-(2-chloro-1-hydroxyl-ethyl)-1,3-dihydro-indol-2-one or 5- (2-Bromo-1-hydroxy-ethyl)-1,3-dihydro-indol-2-one;
[0073] 3) 5-(2-chloro-1-hydroxyl-ethyl)-1,3-dihydro-indol-2-one or 5-(2-bromo-1-hydroxyl-ethyl) obtained in the above steps -1,3-dihydro-indol-2-one, 3-piperazinyl-1,2-benzisothiazole hydrochloride, alkali and the thi...
Embodiment 1
[0113] step 1:
[0114]Add 100mL of dichloromethane into a 250mL three-necked flask, cool to 0°C in an ice-water bath, slowly add aluminum trichloride (30.0g, 225.3mmol) in batches while stirring, keep the temperature below 20°C, a yellow suspension, add Finished, nitrogen protection. Start to slowly add chloroacetyl chloride (10.2 g, 90.1 mmol) dropwise, keeping the temperature below 20°C. After the dropwise addition was completed, stir for 30 min. Compound 2 (10.0 g, 75.1 mmol) was added slowly, the temperature rose to 30° C. during the addition, and the yellow suspension turned into a black solution. Stir under nitrogen protection at room temperature for 24 hours. TLC monitors that the reaction raw materials have completely reacted, and slowly pours into 200 mL of ice water, keeping the temperature below 30°C. A large amount of solids precipitated, and 100 mL of water was added, stirred for 15 minutes, filtered, and the filter cake was slurried with 50 mL of methanol f...
experiment example 2
[0125] The difference from Example 1 is that the equivalent ratio of compound 4 and compound 4a in step 3 is 1:1.0.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com