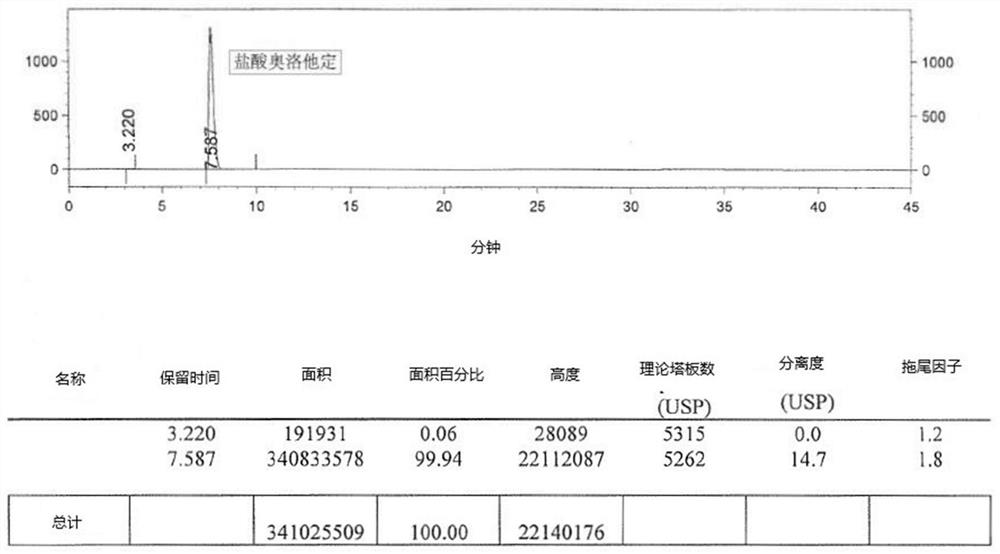
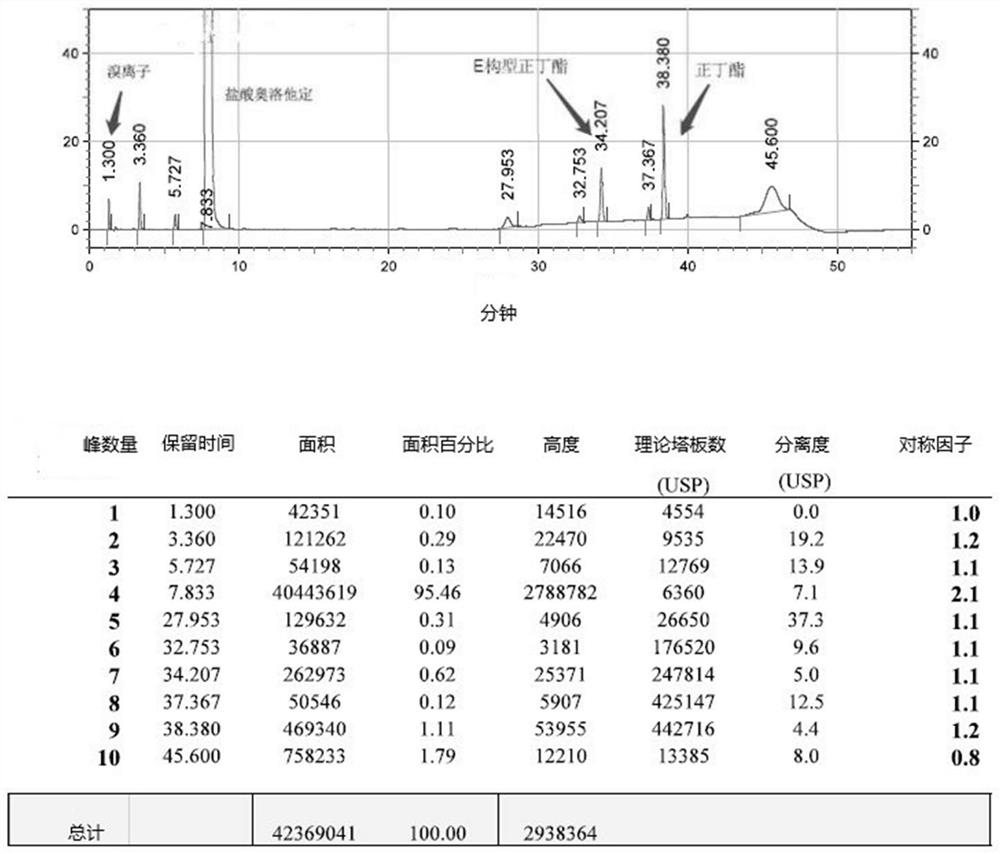
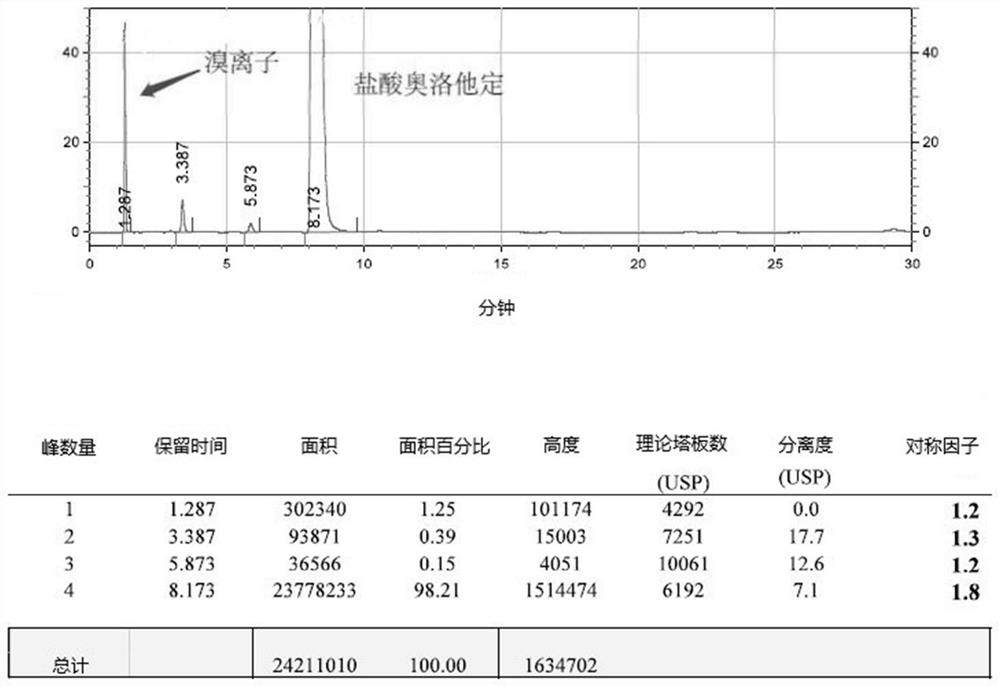
A post-treatment purification method for olopatadine hydrochloride
A technology of olopatadine hydrochloride and a purification method, applied in the field of pharmaceutical purification, can solve the problems of affecting product yield, difficult to remove bromide ion impurities, loss of target product, etc., so as to improve the preparation yield and product purity, and reduce the bromide ion Residual risks, the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The preparation of embodiment 1 olopatadine hydrochloride crude product
[0048] Weigh [3-(dimethylamino)propyl]triphenylphosphine bromide hydrobromide (231.6g) and tetrahydrofuran (915ml) into a reaction flask, nitrogen protection, then stir in a cooling bath at -10°C ; Add BuLi (284.5g) dropwise to the system, keeping the temperature of the system at -10-10°C. After the drop is complete, stir for 2h; add dropwise a solution of isoket acid (61g) in tetrahydrofuran (305ml), and heat up to 55-60°C , Reaction 4h.
[0049] The reaction solution system was lowered to room temperature, and water (305ml) was added dropwise to quench the reaction; then NaCl (91.5g), NaOH (37g), and water (183ml) were added, and the temperature was raised to 55-60°C and stirred for 2h; the phases were separated, and the organic phase was reserved. Add 25% (w / w) sodium chloride solution (305ml) for washing, stir at 35-40°C for 30min, let stand to separate the phases, and keep the organic phas...
Embodiment 2
[0051] The preparation of embodiment 2 olopatadine hydrochloride crude product
[0052] Weigh [3-(dimethylamino)propyl]triphenylphosphine bromide hydrobromide (231.6g) and tetrahydrofuran (915ml) into a reaction flask, nitrogen protection, then stir in a cooling bath at -10°C ; Add BuLi (284.5g) dropwise to the system, keeping the temperature of the system at -10-10°C. After the drop is complete, stir for 2h; add dropwise a solution of isoket acid (61g) in tetrahydrofuran (305ml), and heat up to 55-60°C , Reaction 4h.
[0053] The reaction solution system was lowered to room temperature, and water (305ml) was added dropwise to quench the reaction; then NaCl (91.5g), NaOH (37g), and water (183ml) were added, and the temperature was raised to 55-60°C and stirred for 2h; the phases were separated, and the organic phase was reserved. Add 27% (w / w) sodium chloride solution (305ml) for washing, stir at 45-50°C for 30min, let stand to separate the phases, and keep the organic phas...
Embodiment 3
[0055] The preparation of embodiment 3 olopatadine hydrochloride crude product
[0056] Weigh [3-(dimethylamino)propyl]triphenylphosphine bromide hydrobromide (231.6g) and tetrahydrofuran (915ml) into a reaction flask, nitrogen protection, then stir in a cooling bath at -10°C ; Add BuLi (284.5g) dropwise to the system, keeping the temperature of the system at -10-10°C. After the drop is complete, stir for 2h; add dropwise a solution of isoket acid (61g) in tetrahydrofuran (305ml), and heat up to 55-60°C , Reaction 4h.
[0057] The reaction solution system was lowered to room temperature, and water (305ml) was added dropwise to quench the reaction; then NaCl (91.5g), NaOH (37g), and water (183ml) were added, and the temperature was raised to 55-60°C and stirred for 2h; the phases were separated, and the organic phase was reserved. Add 20% (w / w) sodium chloride solution (305ml) for washing, stir at 35-40°C for 30min, let stand to separate the phases, and keep the organic phas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com