Method for simply and conveniently preparing high-purity olopatadine hydrochloride intermediate
A hydrobromide, triphenylphosphonium technology, applied in the field of medicinal chemistry, can solve the problems of material waste, personal injury, operator allergies, etc., and achieve the effects of not easily accumulating static electricity, reducing workload, and simplifying production operations.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 27
[0070] Example 27, Example 28, and Example 29 are the data obtained by monitoring the same reaction at different reaction times. Therefore, there is no purity and yield data for Example 27 and Example 28.
Embodiment 30
[0071] Embodiment 30 and embodiment 31 are the data obtained by monitoring different reaction times for the same reaction. Therefore, there are no purity and yield data for Example 30.
[0072] From the results in Table 6, the conversion rate can reach about 96% in about 2 hours of reaction; the reaction can be considered as complete after reaction of 4 hours; there is no significant difference between the time extension of 8 hours and the reaction of 4 hours. Considering the above reasons, the reaction time should be between 3 and 5 hours.
Embodiment 32
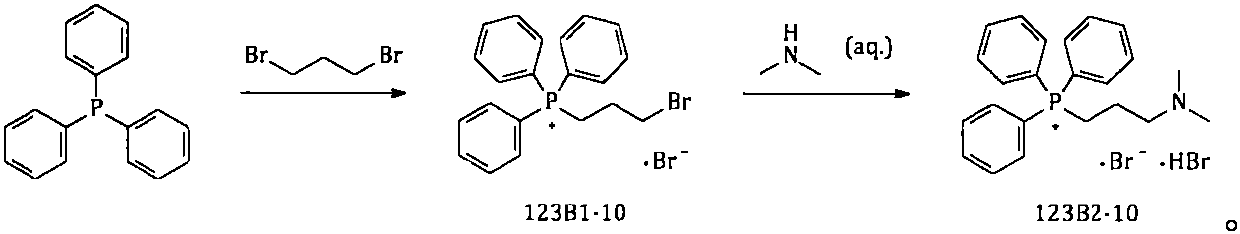
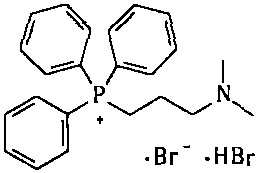
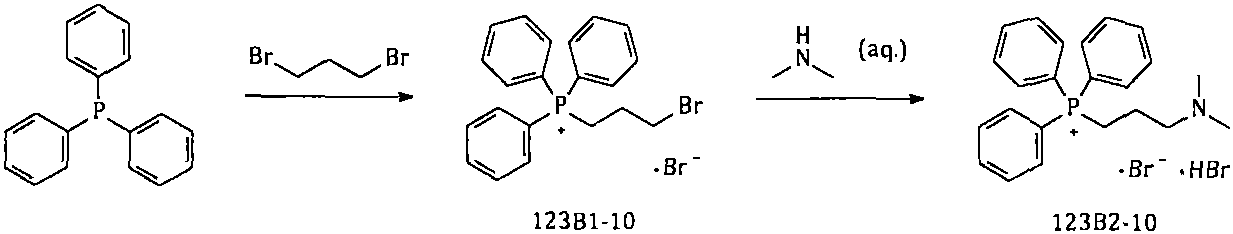
[0081] Example 32. Synthesis of [3-(dimethylamino)propyl]triphenylphosphonium bromide hydrobromide
[0082]
[0083] Add 750kg of n-heptane into a 2000L reactor; then add 250kg of triphenylphosphine; 192kg of 1,3-dibromopropane; heat to reflux and react for about 60hr. After the reaction is complete, cool the reaction solution to 20-30°C; add 335 kg of 40% dimethylamine aqueous solution; raise the temperature to 45-50°C, and keep the reaction for about 5 hours; the solid in the reaction system gradually dissolves completely. After the reaction is completed, concentrate under reduced pressure, collect the azeotrope of n-heptane / water, add the separated n-heptane to the reaction system, and continue to concentrate until the water in the reaction system is basically concentrated to dryness; the reaction solution is cooled and centrifuged Shake filtration to collect the solids; heat the collected solids with absolute ethanol for beating; cool, centrifuge and shake them to colle...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com