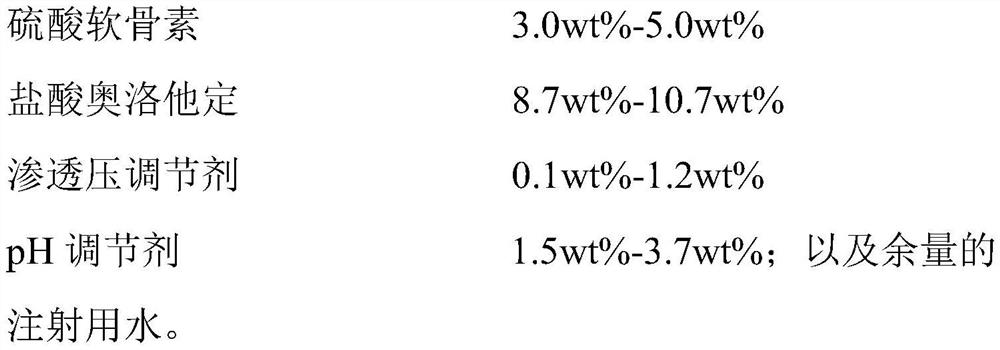Ophthalmic composition as well as preparation method and application thereof
An ophthalmic composition and drug technology, applied in the field of medicine, can solve problems affecting drug compliance, prolong treatment time, accelerate wound healing, etc., achieve the effects of shortening treatment and recovery time, improving comfort, and increasing drug compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1) Preparation of ophthalmic composition
[0035] Step S1, uniformly mixing sodium citrate, sodium chloride and water for injection to prepare a mixed solution I;
[0036] Step S2, adding chondroitin sulfate and olopatadine hydrochloride to water for injection, and stirring homogeneously at 3000r / min for 10min to prepare mixed solution II;
[0037] Step S3, mixing the mixed solution I and the mixed solution II evenly, adding water for injection, filtering through a 0.22 μm filter element, and filling to obtain an ophthalmic composition with the following composition based on the total weight of the ophthalmic composition:
[0038] and the remainder of
[0039] Water for Injection;
[0040] The mass ratio of chondroitin sulfate and olopatadine hydrochloride in the ophthalmic composition is 0.34:1;
[0041] The ratio of the amount of water for injection in step S1, the amount of water for injection in step S2 and the amount of water for injection in step S3 is 3:3:4...
Embodiment 2
[0049] (1) Preparation of ophthalmic composition
[0050] Step S1, uniformly mixing borax, potassium chloride and water for injection to prepare mixed solution I;
[0051] Step S2, adding chondroitin sulfate and olopatadine hydrochloride to water for injection, and stirring homogeneously at 4500r / min for 15min to prepare mixed solution II;
[0052] Step S3, mixing the mixed solution I and the mixed solution II evenly, adding water for injection, filtering through a 0.22 μm filter element, and filling to obtain an ophthalmic composition with the following composition based on the total weight of the ophthalmic composition:
[0053] and the remainder of
[0054] Water for Injection;
[0055] The mass ratio of chondroitin sulfate and olopatadine hydrochloride in the ophthalmic composition is 0.28:1;
[0056] The ratio of the amount of water for injection in step S1, the amount of water for injection in step S2 and the amount of water for injection in step S3 is 2:5:3.
[00...
Embodiment 3
[0063] (1) Preparation of ophthalmic composition
[0064]The preparation method of the ophthalmic composition of this embodiment is the same as that of Example 1, the difference is that the osmotic pressure regulator is glycerin, and the pH regulator is citric acid, and the following formula is obtained based on the total weight of the ophthalmic composition: Composition of the ophthalmic composition:
[0065] and the remainder of
[0066] Water for Injection;
[0067] The mass ratio of chondroitin sulfate to olopatadine hydrochloride in the ophthalmic composition is 0.41:1.
[0068] (2) Performance analysis of eye drops
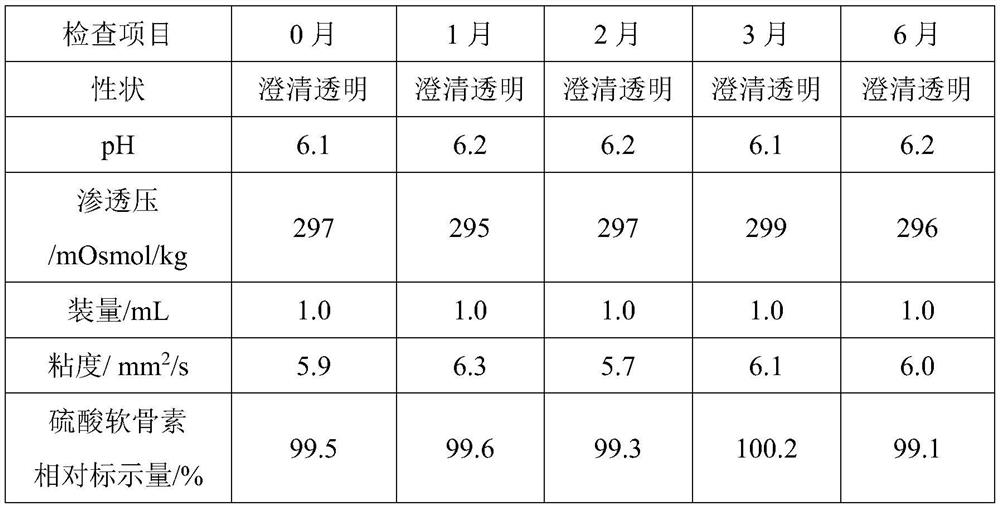
[0069] The ophthalmic composition prepared in Example 3 was used as eye drops, and the accelerated stability inspection was carried out according to the 2015 edition of "Chinese Pharmacopoeia" 9001 preparation stability inspection principles, and the inspection was carried out according to the indicators under the 0105 ophthalmic preparation general rul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com