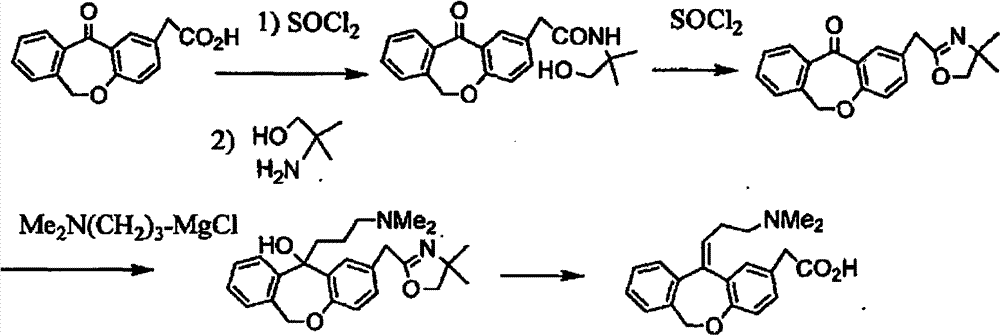
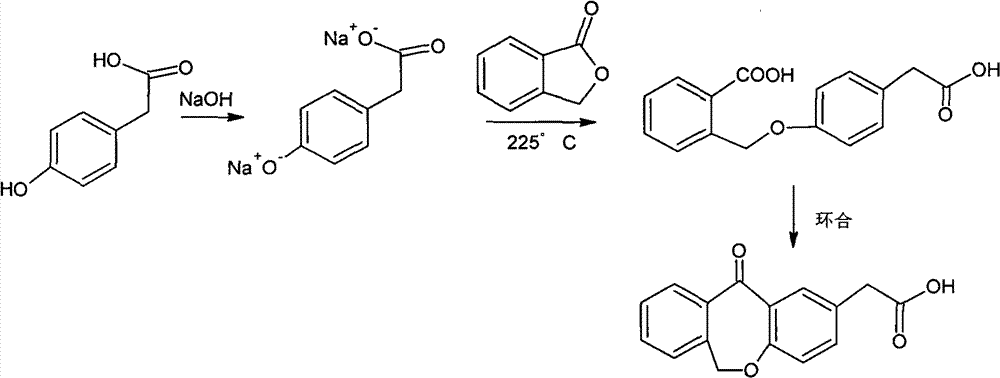
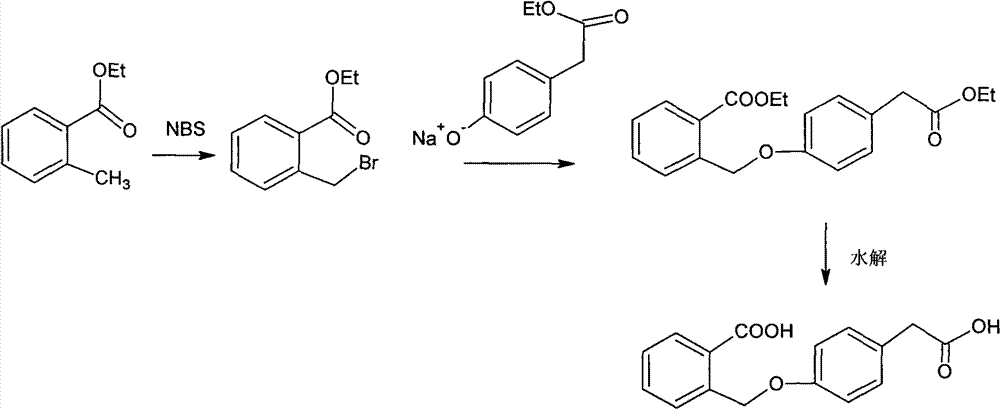
Improved preparation method of 4-(2-carboxybenzyloxy) phenylacetic acid
A technology of carboxybenzyloxy and hydroxyphenylacetic acid, applied in the preparation of carboxylate/lactone, organic chemistry, etc., can solve the problems of many steps, poor safety, low yield, etc., shorten the process steps and improve safety , the effect of reducing energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 8
[0058] Embodiment one to eight 4-(2-carboxybenzyloxy) phenylacetic acid preparation
[0059] Dissolve phthalide in dimethyl sulfoxide and toluene, it can be completely dissolved at about 40°C, and set aside.
[0060] Add 4-hydroxyphenylacetic acid to dimethyl sulfoxide, stir, and when the internal temperature is 48°C, add 50% sodium hydroxide aqueous solution dropwise, the reaction is exothermic, control the temperature below 95°C to prevent flushing; then add toluene , heating and reflux began to separate water. After the water separation is complete, steam the toluene, add about half of the pre-prepared phthalide solution directly, steam the toluene, heat to 125-140°C for reaction, and add the other half of the phthalide after the phthalide is basically completely reacted Solution, distill out toluene, heat to 125-140°C for reaction, and react until all phthalides have reacted. Cool the reaction system to about 100°C, add water, and when the temperature drops to room tempe...
Embodiment 9 10
[0065] Embodiment nine to sixteen 4-(2-carboxybenzyloxy) phenylacetic acid preparation
[0066] Dissolve phthalide in dimethyl sulfoxide and xylene, it can be completely dissolved at about 40°C, and set aside.
[0067] Add 4-hydroxyphenylacetic acid to dimethyl sulfoxide, stir, and when heated to an internal temperature of 48°C, add 50% sodium hydroxide aqueous solution dropwise, the reaction is exothermic, and the temperature is controlled below 95°C to prevent flushing; then add two Toluene, the temperature was raised to reflux and the water was separated. After the water separation is complete, distill out the xylene, directly add about half of the pre-prepared phthalide solution, steam the xylene, heat to 140-150°C for reaction, and add the other half after the phthalide basically reacts completely For phthalide solution, distill out xylene, heat to 140-150°C for reaction, and react until all phthalide has reacted. Cool the reaction system to about 100°C, add water, and ...
Embodiment 17
[0070] Example 17 Preparation of 6,11-dihydro-11-oxo-dibenzo[b,e]oxepadiene-2-acetic acid
[0071] Under stirring, add 28.6g of 4-(2-carboxybenzyloxy)phenylacetic acid, 100g of polyphosphoric acid, and 100ml of glacial acetic acid into the reaction bottle, heat to 80°C for 5 hours, after the reaction is complete, pour the reaction solution into cold water , extracted with 100ml×3 ethyl acetate, washed with 200ml×3 water to weak acidity, after liquid separation, added 3g of activated carbon to the ethyl acetate layer for decolorization, heated to reflux for 30 minutes, and filtered off the activated carbon while hot. Dry over anhydrous sodium sulfate, filter off the desiccant, evaporate ethyl acetate under reduced pressure, and then recrystallize with 65ml 1:1 (v / v) ethyl acetate-n-hexane to obtain 6,11-dihydro-11-oxo- Dibenzo[b,e]oxepadiene-2-acetic acid. After drying, 15.6 g of a light yellow to off-white solid was obtained, with a yield of 58%, a melting point of 137-139° C...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com