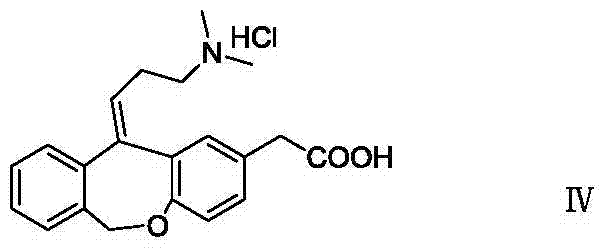
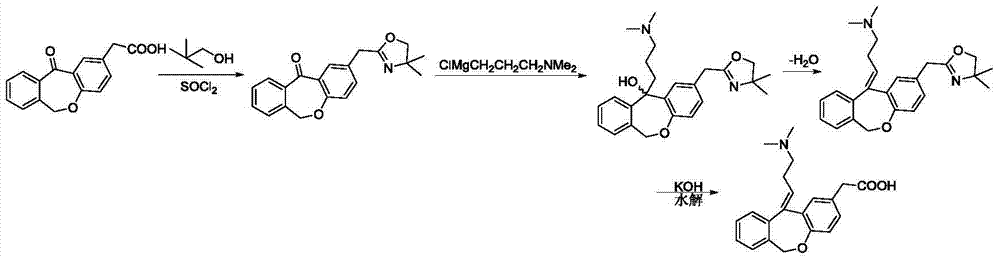
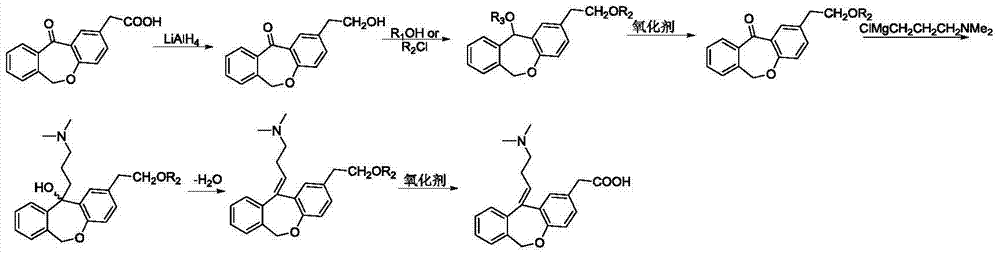
Novel method for preparing olopatadine hydrochloride by using high-activity organic zinc reagent
A technology of olopatadine and high-activity zinc, which is applied in the field of preparing olopatadine hydrochloride with high-activity organozinc reagent, can solve the problems of complicated post-processing, long process steps, etc., and achieves simplified post-processing process, simple operation and product high purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Preparation of Highly Active Organozinc Reagent
[0036] Under the protection of nitrogen, add 3-bromo-N,N-dimethylpropanamine (5.0g, 30mmol) and 32ml of anhydrous tetrahydrofuran to the reaction flask, stir and cool to 10-20℃, add activated zinc powder (3.9 g, 60mmol), after the addition, the temperature was controlled at 20-30°C and stirred for 0.5h, heated to reflux (65-70°C), and stirred for 5-6h. The resulting reaction solution is a highly active organic zinc reagent.
[0037] Preparation of Crude Olopatadine
[0038] Dissolve isoket acid (4.0g, 15mmol) in 12ml of tetrahydrofuran, stir, dissolve and clarify, then drop into the above-mentioned highly active organic zinc reagent at a temperature of 0-10°C, after about 0.5h, raise the temperature to 20-25°C and stir to react After 16 hours, HPLC tracked the end point of the reaction, and the purity was 37.6%.
[0039] Cool the above reaction solution, add 25ml of water for extraction, adjust the pH of the water pha...
Embodiment 2
[0043] Preparation of lithium naphthalene reagent
[0044] Under the protection of nitrogen, lithium metal (0.4 g, 60 mmol) was added to anhydrous naphthalene (2.6 g, 20 mmol), and the temperature was controlled at 20-25° C. and stirred for 2 h to obtain lithium naphthalene reagent.
[0045] Preparation of Highly Active Organozinc Reagent
[0046] Under the protection of nitrogen, add 3-bromo-N,N-dimethylpropanamine (5.0g, 30mmol) and 32ml of anhydrous tetrahydrofuran to the reaction flask, stir and cool to 10-20℃, add zinc bromide (13.5 g, 60 mmol), stirred for 10 min, added dropwise the above-mentioned naphthalene lithium reagent, after the reaction temperature was stabilized, heated to reflux (65-70°C), and stirred for 5-6 h. The resulting reaction solution is a highly active organic zinc reagent.
[0047] Preparation of Crude Olopatadine
[0048] Dissolve isoket acid (4.0g, 15mmol) in 12ml of tetrahydrofuran, stir to dissolve and clarify, then drop into the above-mentio...
Embodiment 3
[0053] Preparation of Highly Active Organozinc Reagent
[0054] Under the protection of nitrogen, add 3-bromo-N,N-dimethylpropylamine (5.0g, 30mmol), zinc bromide (13.5g, 60mmol) and 32ml of anhydrous tetrahydrofuran into the reaction flask, stir and cool to 10-20 ℃, add metal lithium (0.4g, 60mmol) in batches, after the reaction temperature stabilizes, heat to reflux (65-70℃), and stir for 5-6h. The obtained reaction solution is the highly active organic zinc reagent.
[0055] Preparation of Crude Olopatadine
[0056]Dissolve isoket acid (4.0g, 15mmol) in 12ml of tetrahydrofuran, stir, dissolve and clarify, then drop into the above-mentioned highly active organic zinc reagent at a temperature of 0-10°C, after about 0.5h, raise the temperature to 20-25°C and stir to react After 16 hours, HPLC tracked the end point of the reaction, and the purity was 75.2%.
[0057] Cool the above reaction solution, add 25ml of water for extraction, adjust the pH of the water phase to 4.0-4....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com