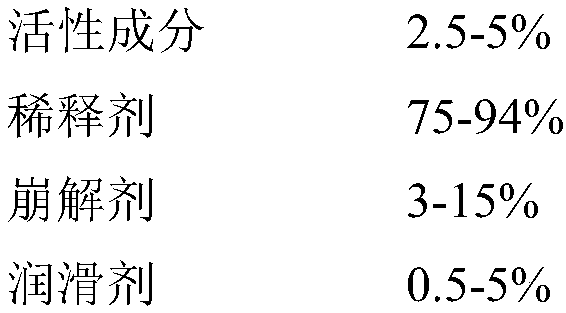
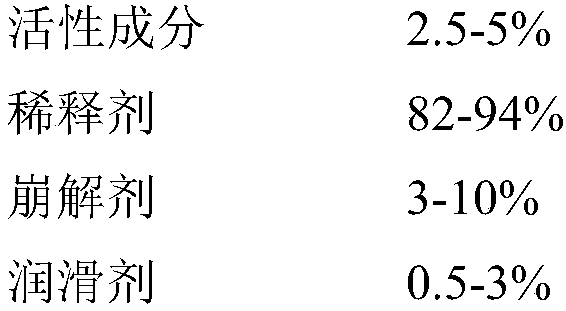
Formula of directly tableted olopatadine hydrochloride tablet
A technology of olopatadine hydrochloride and tablets, which is applied in the field of medicine and can solve the problems of limited physical and chemical properties of active ingredients, easy adsorption, and dissolution resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: the olopatadine hydrochloride tablet formula of direct compression
[0023] In this example, olopatadine hydrochloride with an average particle size of 43 μm is used. Table 1 lists the formulation information of the tablet and the batch size of 1000 tablets.
[0024] Table 1
[0025] Element effect weight percentage (%) Batch (g) Olopatadine Hydrochloride active ingredient 3.0 5.0 microcrystalline cellulose Thinner 7.7 12.9 lactose Thinner 77.3 129.0 Low-substituted hydroxypropyl cellulose disintegrant 5.0 8.4 Sodium carboxymethyl starch disintegrant 5.0 8.4 Magnesium stearate lubricant 2.0 3.3
[0026] The active ingredient, the diluent and the disintegrating agent are mixed, the mixture is sieved, the sieved materials are mixed, a lubricant is added for total blending, and the total blended materials are pressed into tablets.
Embodiment 2
[0027] Embodiment 2: the olopatadine hydrochloride tablet formula of direct compression
[0028] In this example, olopatadine hydrochloride with an average particle size of 9 μm is used. Table 2 lists the formulation information of the tablet and the batch size of 1000 tablets.
[0029] Table 2
[0030] Element effect weight percentage (%) Batch (g) Olopatadine Hydrochloride active ingredient 4.0 5.0 microcrystalline cellulose Thinner 18.0 22.5 lactose Thinner 72.0 90.0 Croscarmellose Sodium disintegrant 5.0 6.3 Magnesium stearate lubricant 1.0 1.2
[0031] Tablets were prepared according to the method of Example 1.
Embodiment 3
[0032] Embodiment 3: the olopatadine hydrochloride tablet formula of direct compression
[0033] In this example, olopatadine hydrochloride with an average particle size of 50 μm is used. Table 3 lists the formulation information of the tablet and the batch size of 1000 tablets.
[0034] table 3
[0035] Element effect weight percentage (%) Batch (g) Olopatadine Hydrochloride active ingredient 3.5 5.0 microcrystalline cellulose Thinner 42.3 60.4 lactose Thinner 42.2 60.4 Crospovidone disintegrant 5.0 7.2 Low-substituted hydroxypropyl cellulose disintegrant 3.0 4.3 Magnesium stearate lubricant 1.0 1.4 Sodium stearyl fumarate lubricant 3.0 4.3
[0036] Tablets were prepared according to the method of Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com