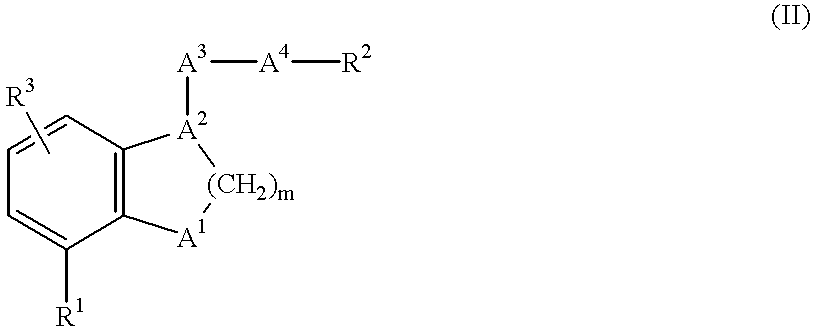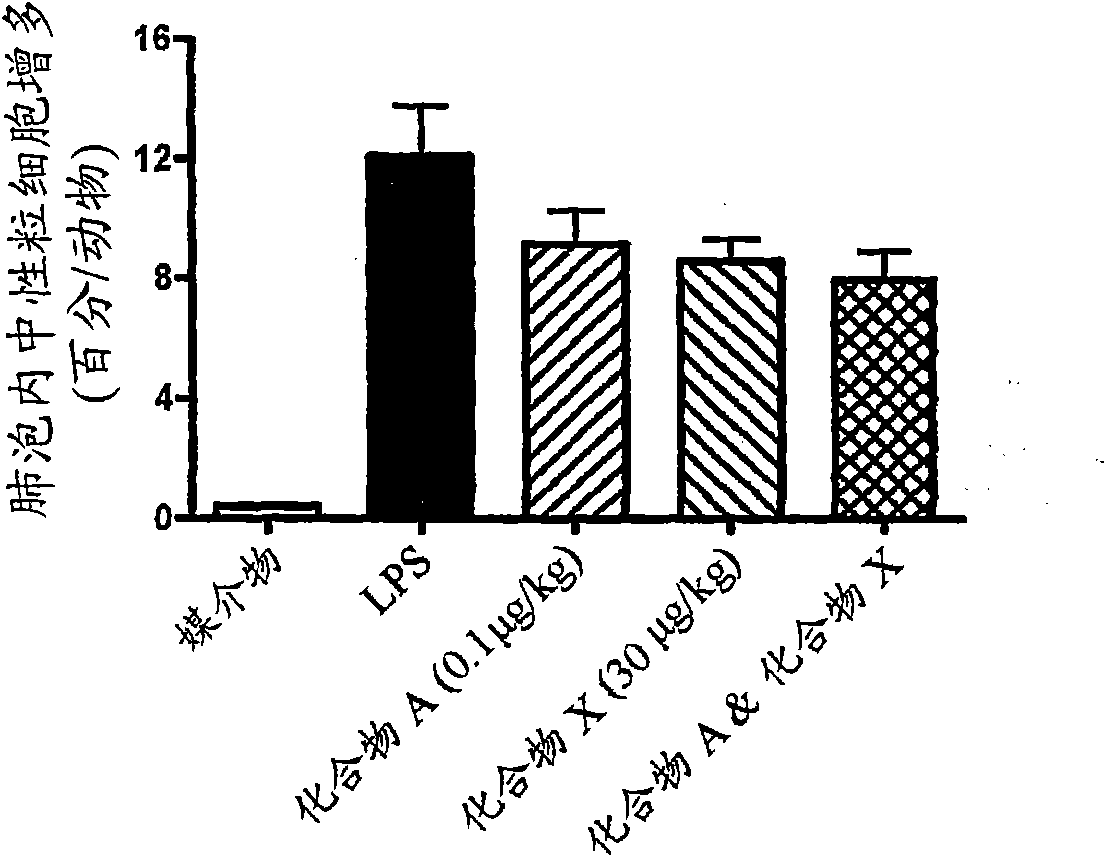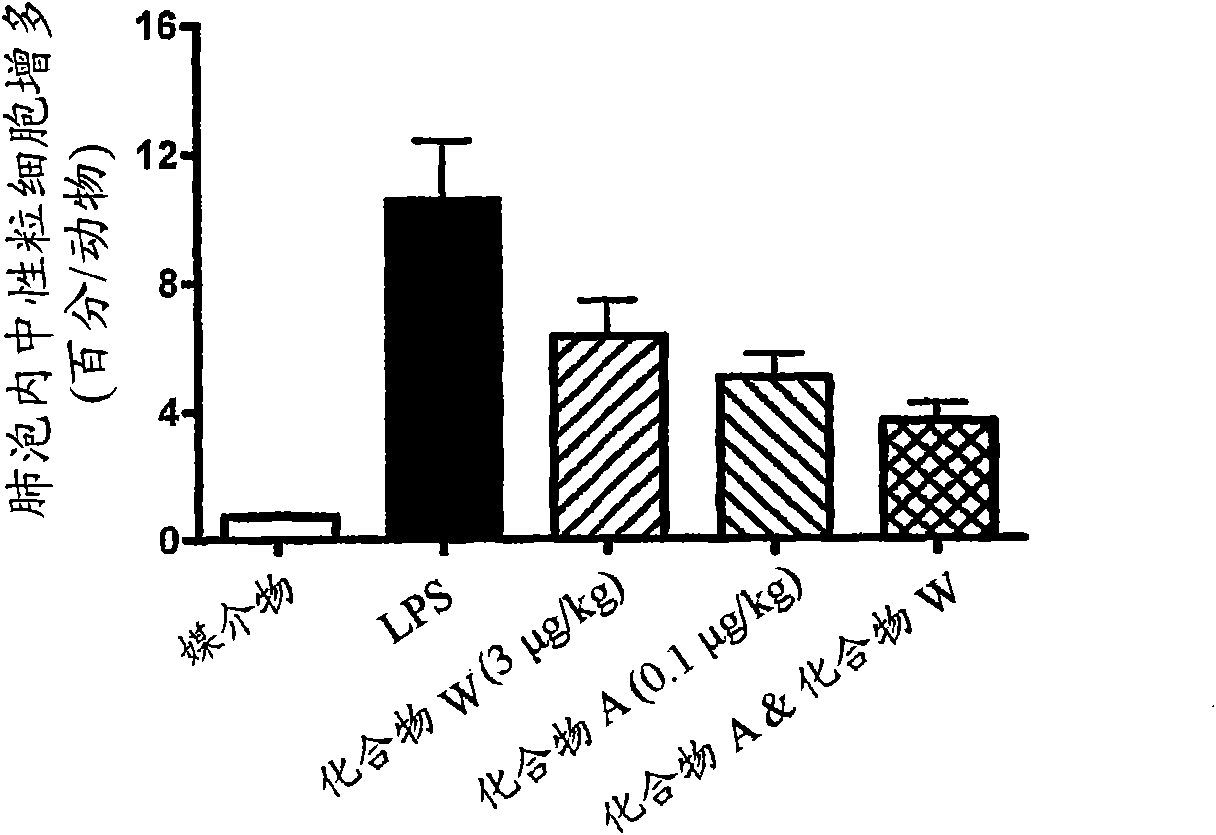Patents
Literature
40 results about "Thromboxane" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Thromboxane is a member of the family of lipids known as eicosanoids. The two major thromboxanes are thromboxane A2 and thromboxane B2. The distinguishing feature of thromboxanes is a 6-membered ether-containing ring.
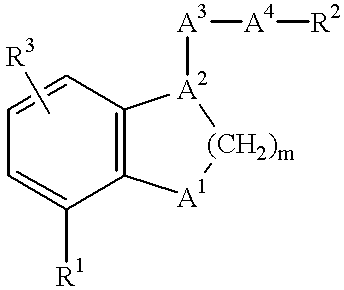
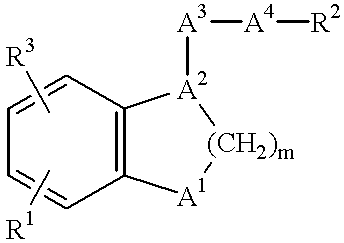
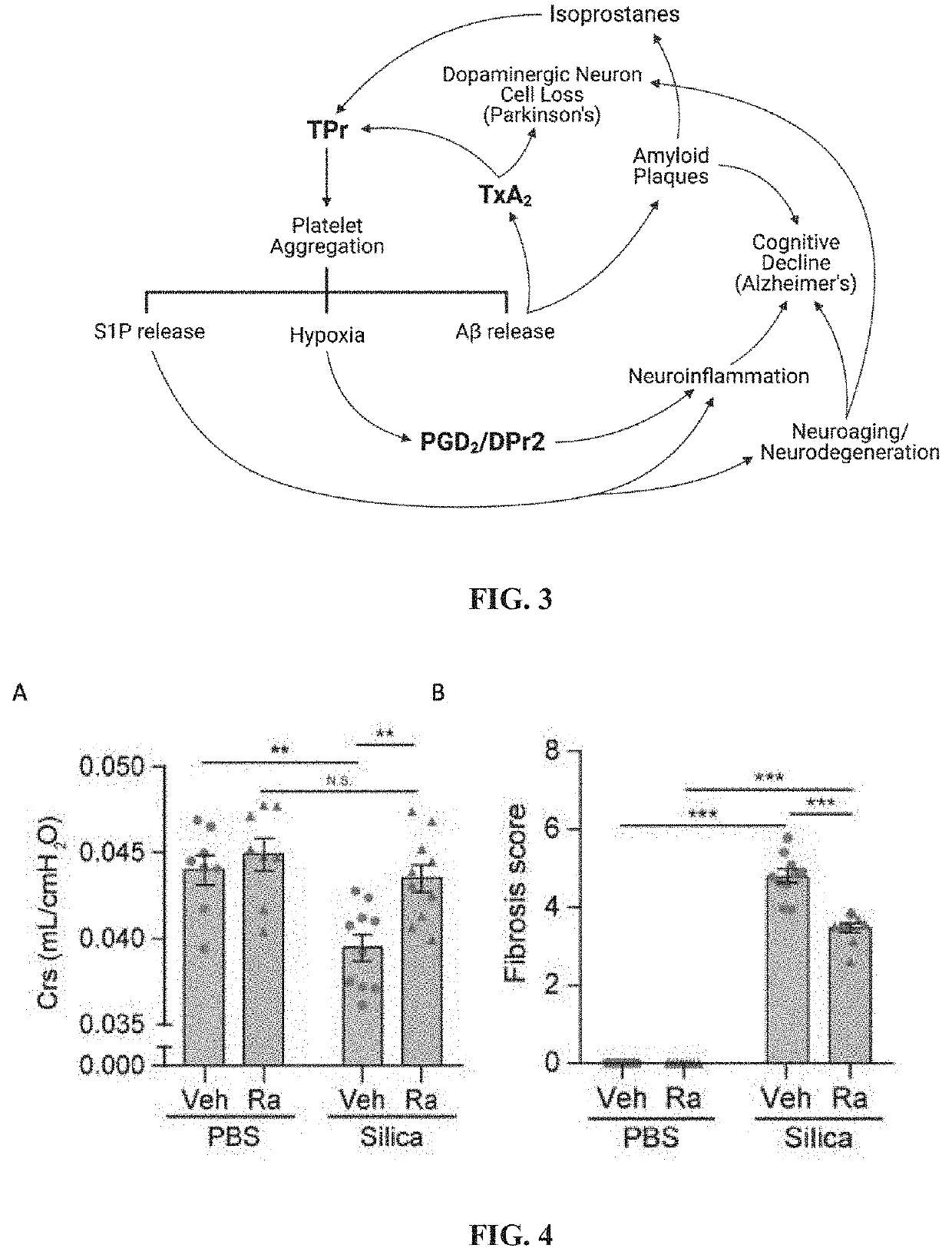
Pharmaceutical composition comprising a dual antagonist against PGD2/TXA2 receptors having a [2.2.1] or [3.1.1] bicyclic skeleton
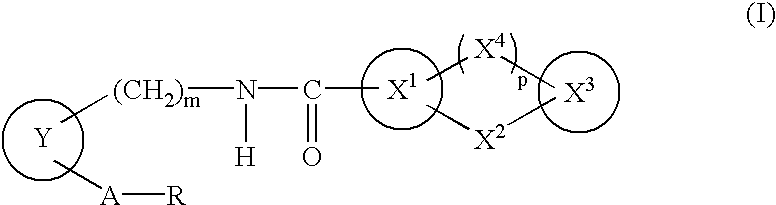
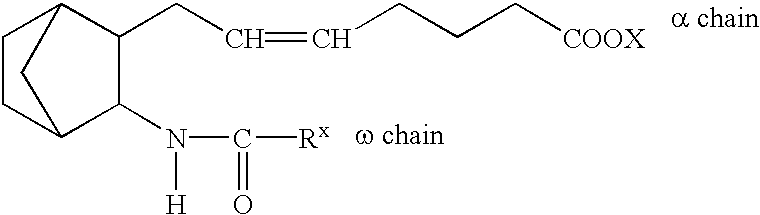
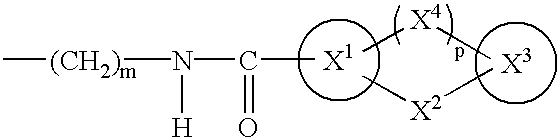
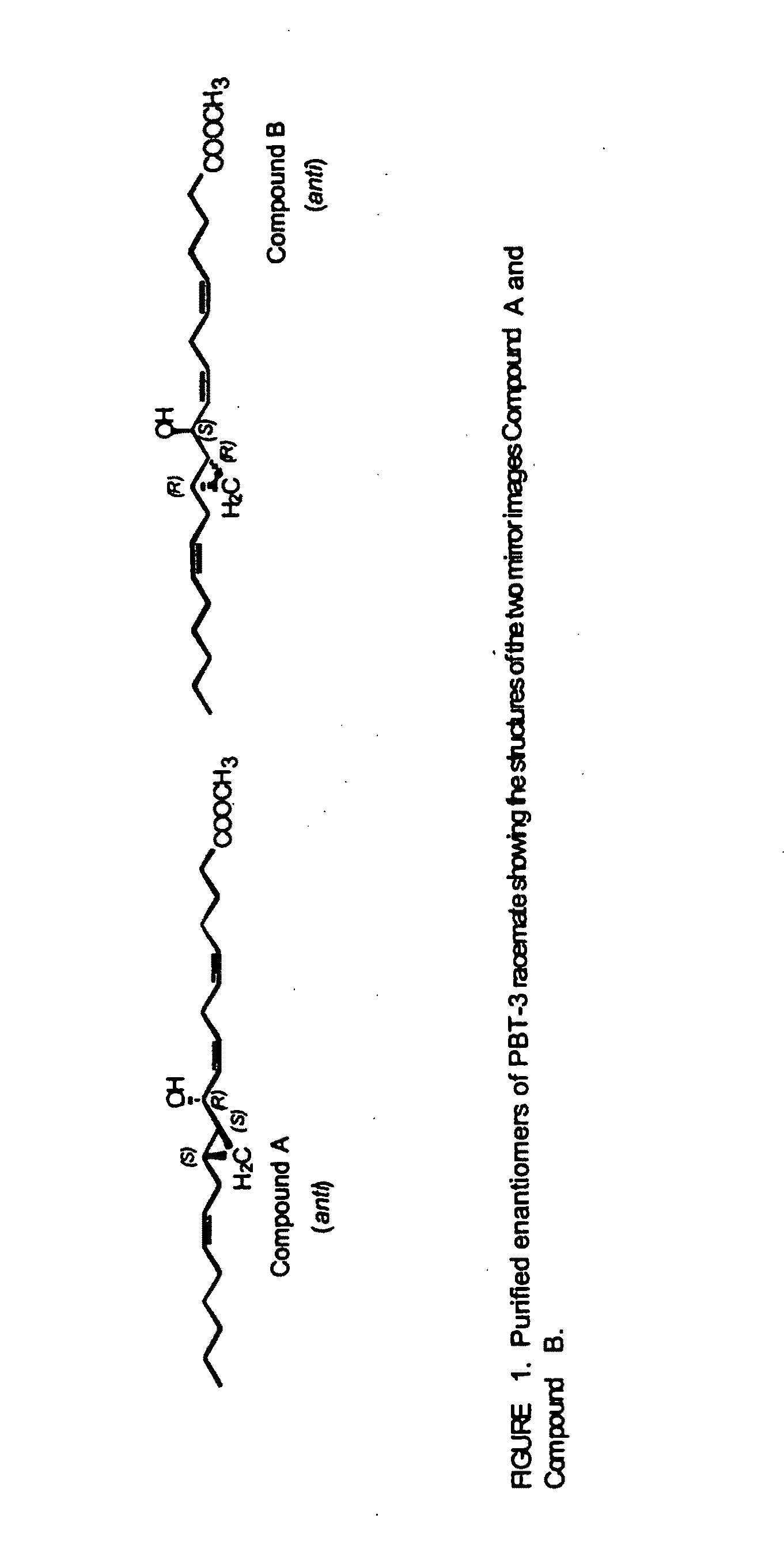
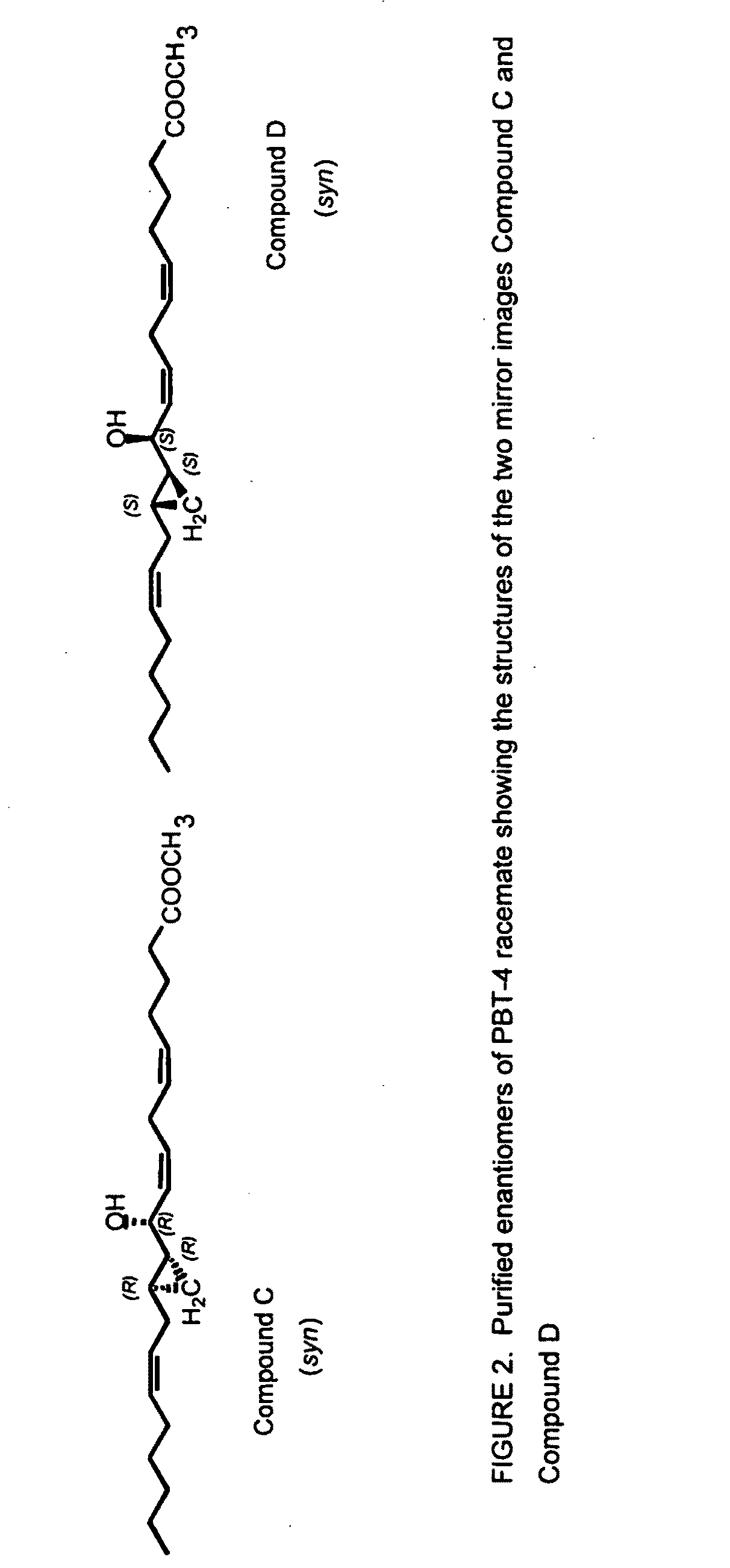
The present invention provides novel compounds having a dual antagonistic activity against thromboxane A2 receptor and prostaglandin D2 receptor and pharmaceutical compositions comprising them.A compound of the formula (I):wherein R1 is —CH2—CH═CH—CH2—CH2—CH2—COOR2 or —CH═CH—CH2—CH2—CH2—COOR2; R2 is hydrogen or alkyl; m is 0 or 1; p is 0 or 1; X1 and X3 each is independently optionally substituted aryl or optionally substituted heteroaryl; X2 is a bond, —CH2—, —S—, —SO2—, —CH2—O—, —O—CH2—, —CH2—S—, —S—CH2—, or the like; X4 is —CH2—, —CH2—CH2—, —C(═O)—, or the like, have a dual antagonistic activity against both a thromboxane A2 receptor and a prostaglandin D2 receptor.
Owner:SHIONOGI & CO LTD
Drug composition antagonistic to both PGD2/TXA2 receptors
A compound of the formula (I):wherein A is alkylene optionally having an unsaturated bond; R is —C(═O)—R1; R1 is hydroxy or the like; m is 0 or 1; p is 0 or 1; X1 and X3 are each independently optionally substituted aryl or optionally substituted heteroaryl or the like; X2 is a bond, —CH2—, —S—, —SO2—, —CH2—O—, —O—CH2—, —CH2—S—, —S—CH2—, or the like; X4 is —CH2—, —CH2—CH2—, —C(═O)—, or the like, having a dual antagonistic activity against both a thromboxane A2 receptor and a prostaglandin D2 receptor is found.
Owner:SHIONOGI & CO LTD
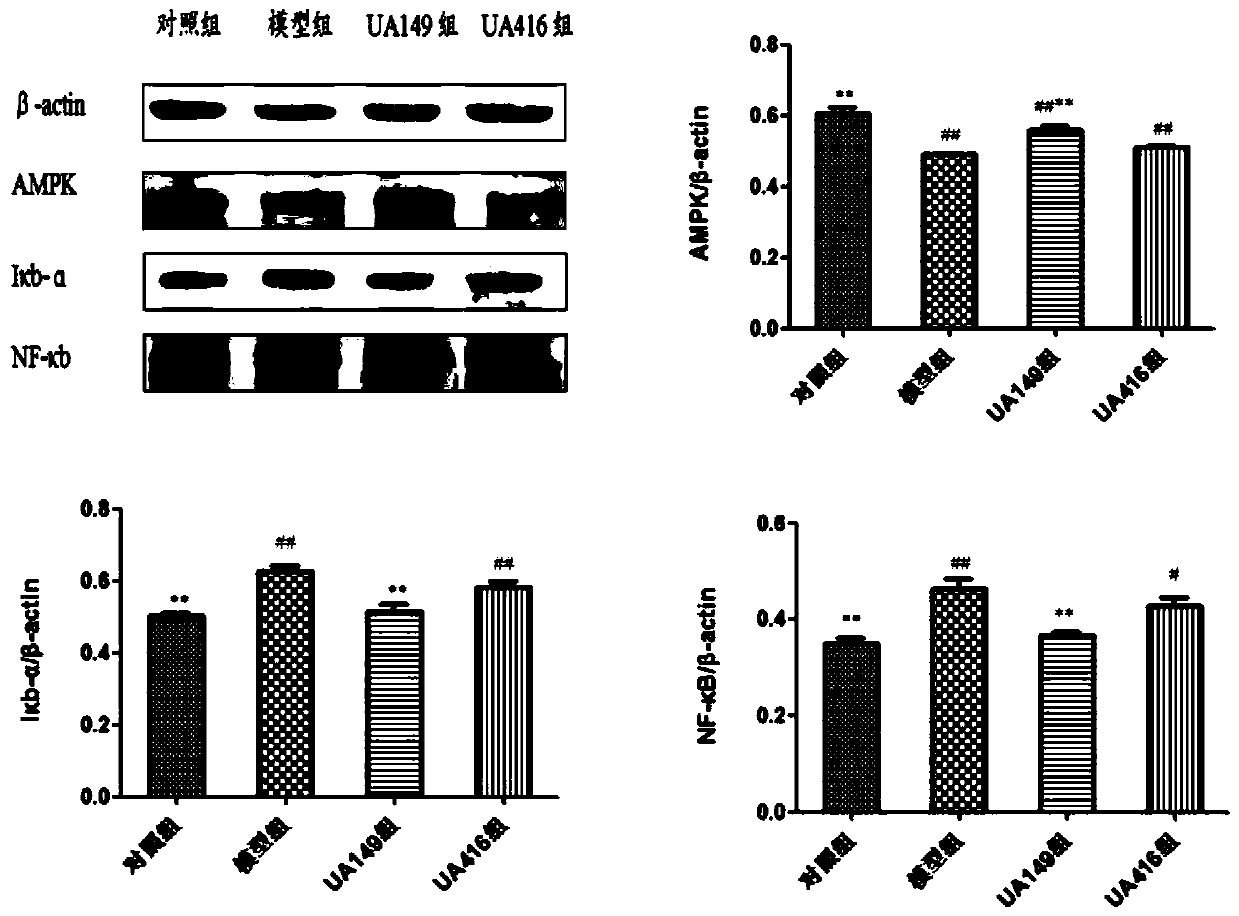
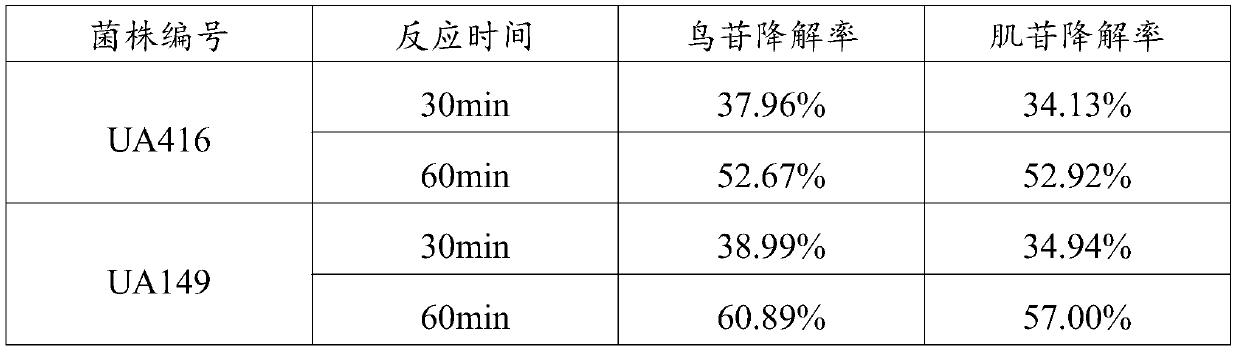
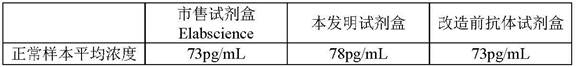
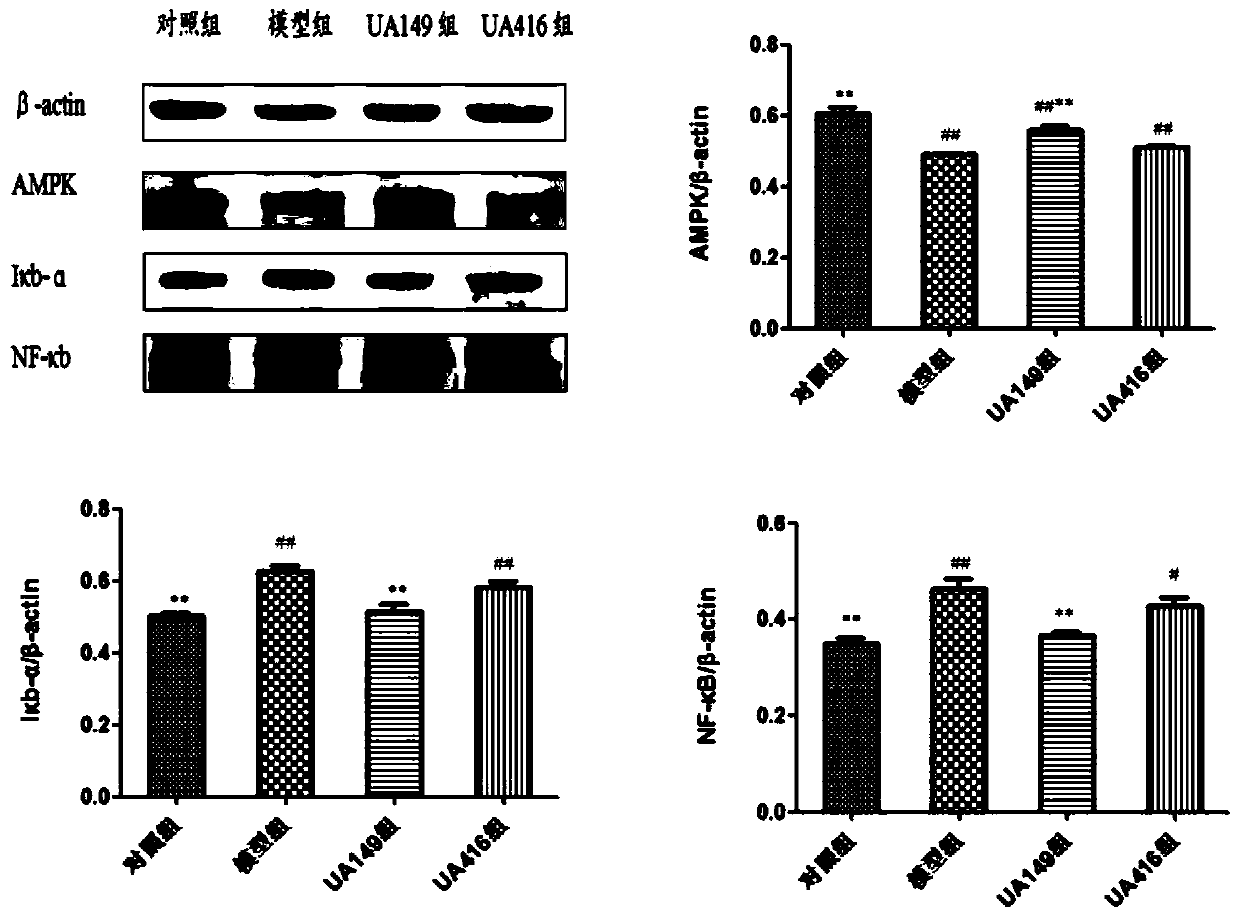
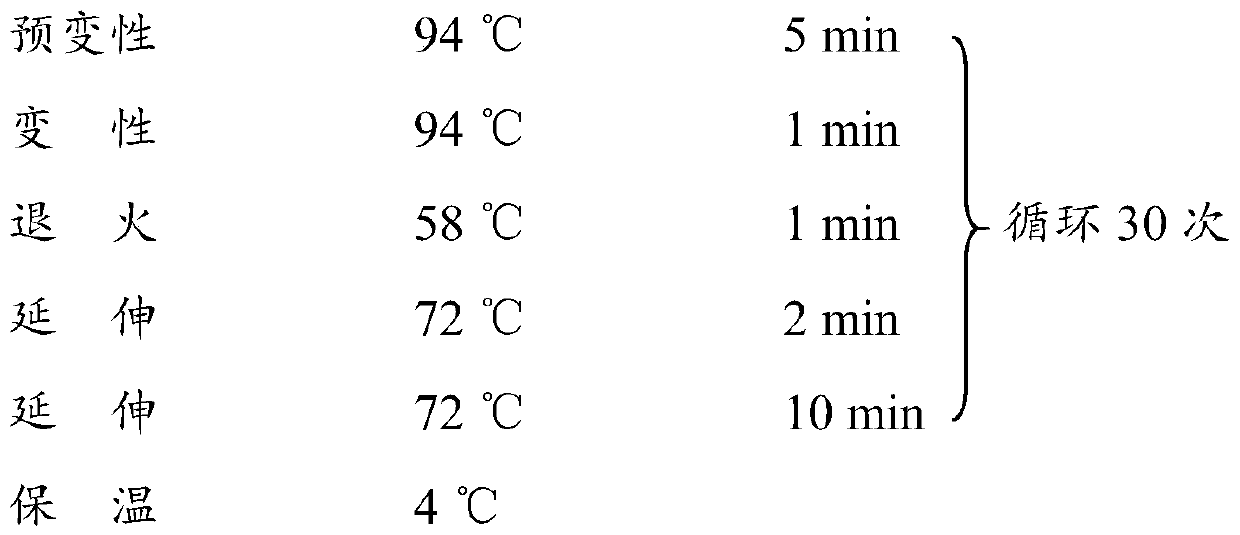
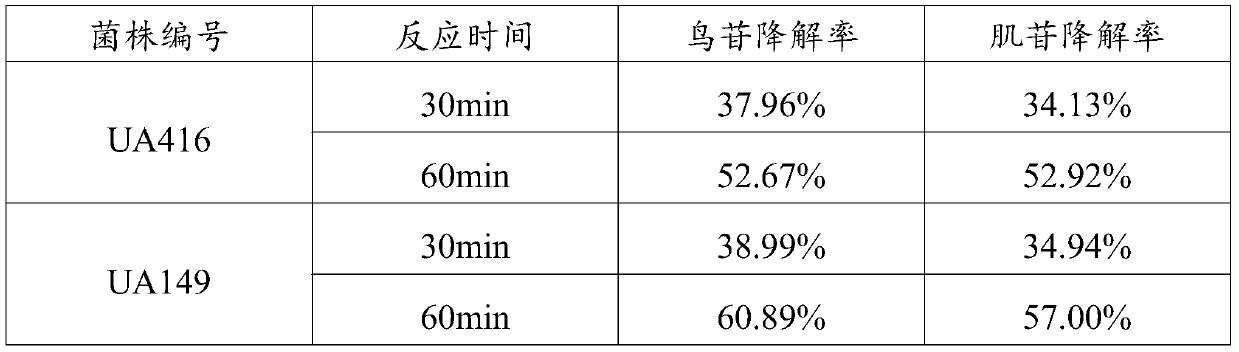
L.plantarum UA149 strain and application thereof
ActiveCN110055199AReduce synthesisLower uric acid levelsBacteriaMicroorganism based processesInflammatory factorsResearch Object
The invention discloses an L.plantarum UA149 strain and an application thereof, and belongs to the field of functional food microorganisms. The L.plantarum UA149 strain is deposited in China Typical Culture Collection Center on November 29, 2018 with the preservation number of CCTCC No: M2018842. The strain can be apply to preparing products with a uric acid reducing function or a gout resisting function. Lactic acid bacteria separated and identified from the surface of fleshy plant leaves are taken as research objects, and a new strain of lactic acid bacteria is screened through a large number of experiments. Hyperuricemia model rats are established by potassium oxazinate combined with fructose water, continuous intragastric administration of the lactobacillus plantarum UA149 strain for 14 days can significantly reduce the level of uric acid; and during gout attack, the release of inflammatory factors thromboxane and leukotriene mediated by neutrophils is reduced, the influx of neutrophils into joints is avoided, and the symptoms of redness, swelling, pain, heat and the like are reduced.
Owner:JILIN MINGZHIYUAN BIOTECH
Benzene fused heterocyclic derivatives having thromboxane A2 receptor antagonistic activity and prostaglandin I2 Agonistic activity and application thereof
InactiveUS6407096B1Stable chemical structureEasy to separateOrganic chemistryHeterocyclic compound active ingredientsBenzeneDisease
Owner:TORAY IND INC
Methods of treating hepatorenal syndrome and hepatic encephalopathy with thromboxane-a2 receptor antagonists
InactiveUS20130197044A1Avoid failureSpeed up the flowBiocideNervous disorderThromboxane A2 receptorNK1 receptor antagonist
The present invention is directed to methods of treating hepatorenal syndrome by administration of a therapeutically effective amount of a thromboxane A2 receptor antagonist to a patient in need thereof. The present invention is also directed to methods of treating hepatic encephalopathy and cerebral edema by administration of a therapeutically effective amount of a thromboxane A2 receptor antagonist to a patient in need thereof.
Owner:CUMBERLAND EMERGING TECH
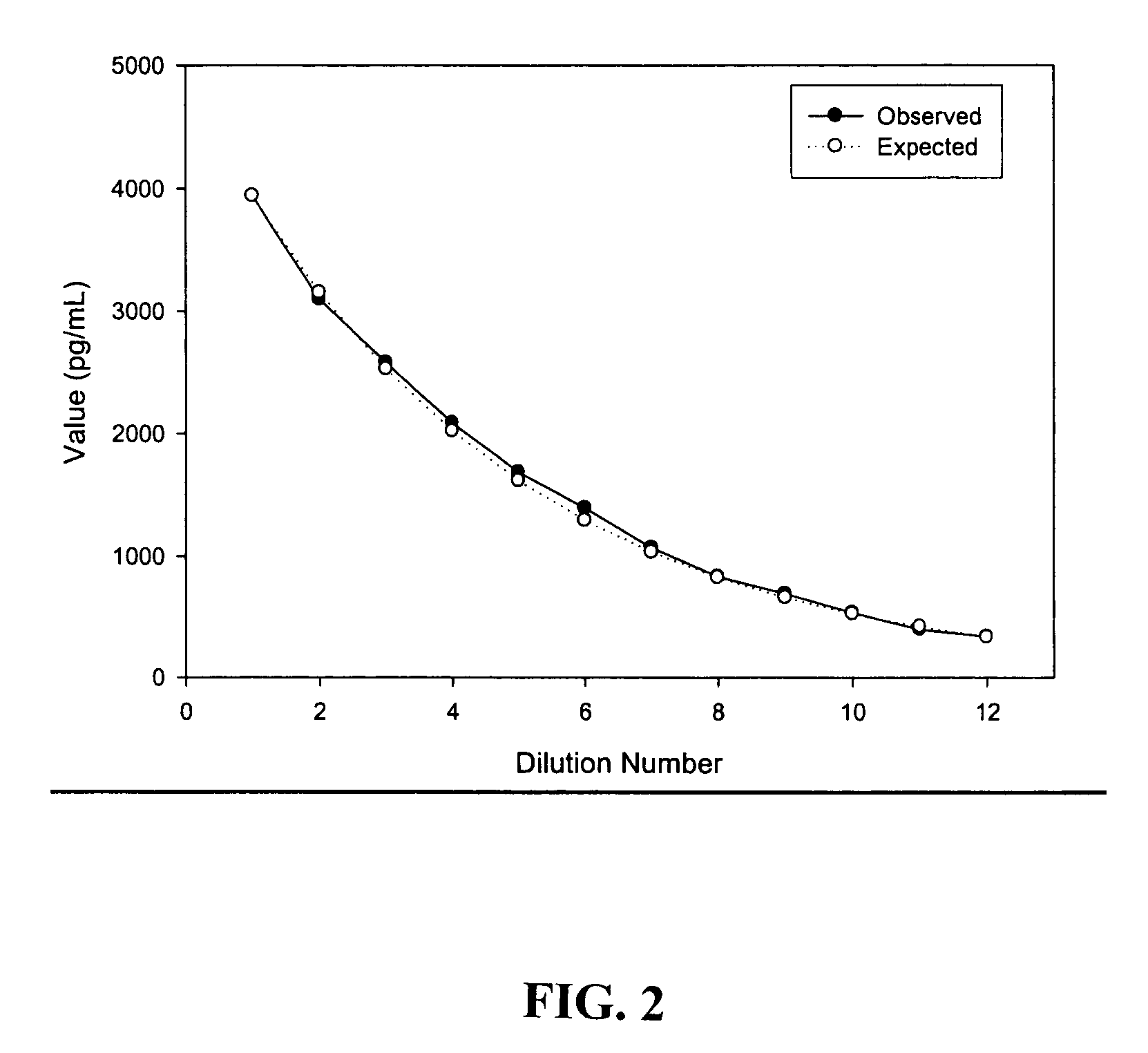
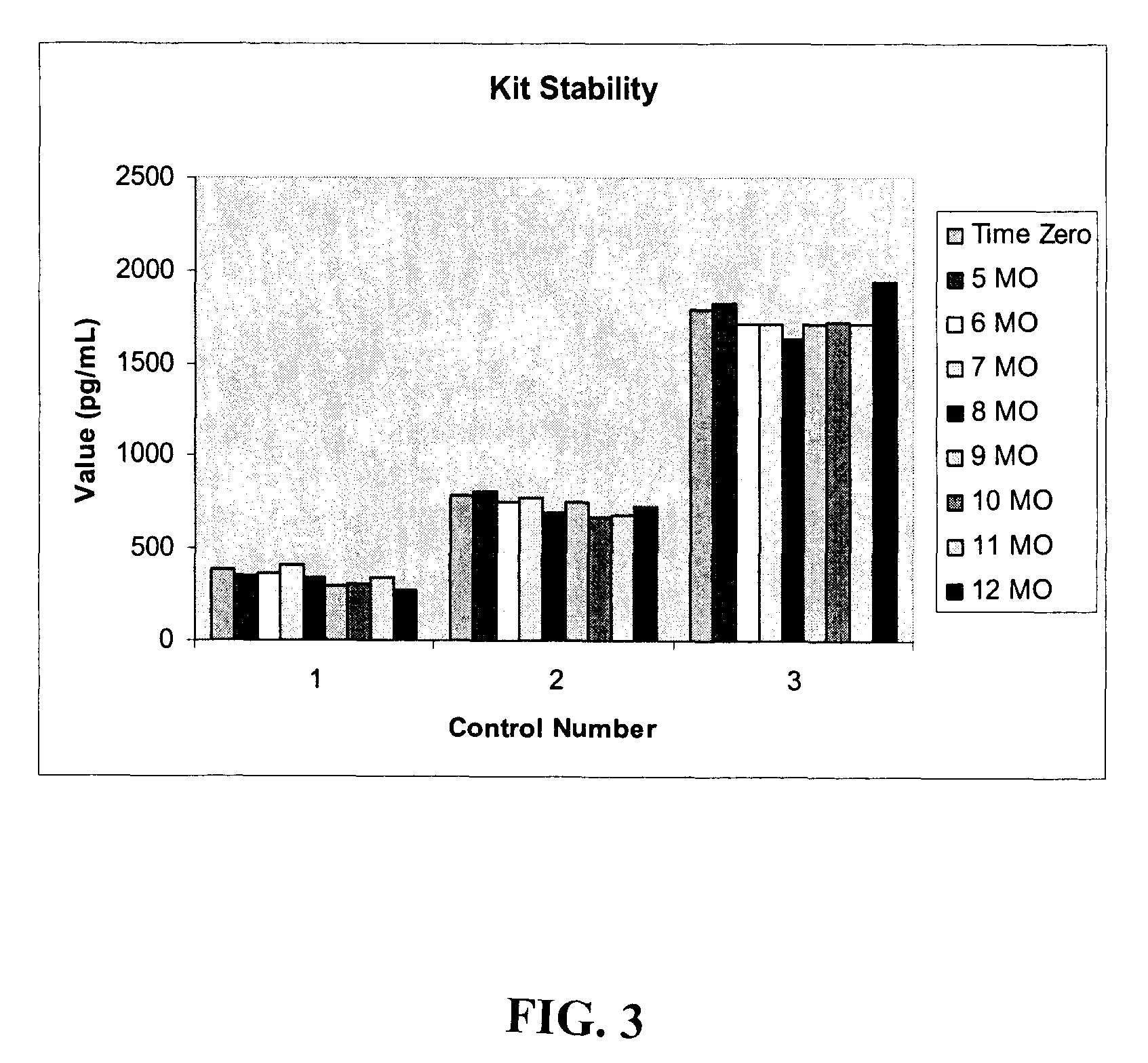
Kit for detecting curative effect of aspirin and detection method thereof
The invention provides a kit for detecting the curative effect of aspirin, belonging to the technical field of biology. The kit comprises a 11-dehydrogenated thromboxane B2 antibody-coated elisa plate, a horseradish peroxidase-marked 11-dehydrogenated thromboxane B2 antibody, 11-dehydrogenated thromboxane B2 standard solution, substrate color developing liquid and an interference shielding agent. The kit for detecting the curative effect of the aspirin comprises the interference shielding agent, so the interference of NaN3 in a detection sample can be effectively avoided, the generation of false negative is reduced, and the detection sensitivity and accuracy for the curative effect of the aspirin are improved.
Owner:华阴市锦前程药业有限公司
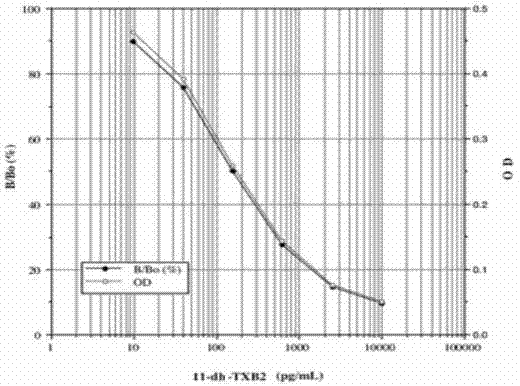
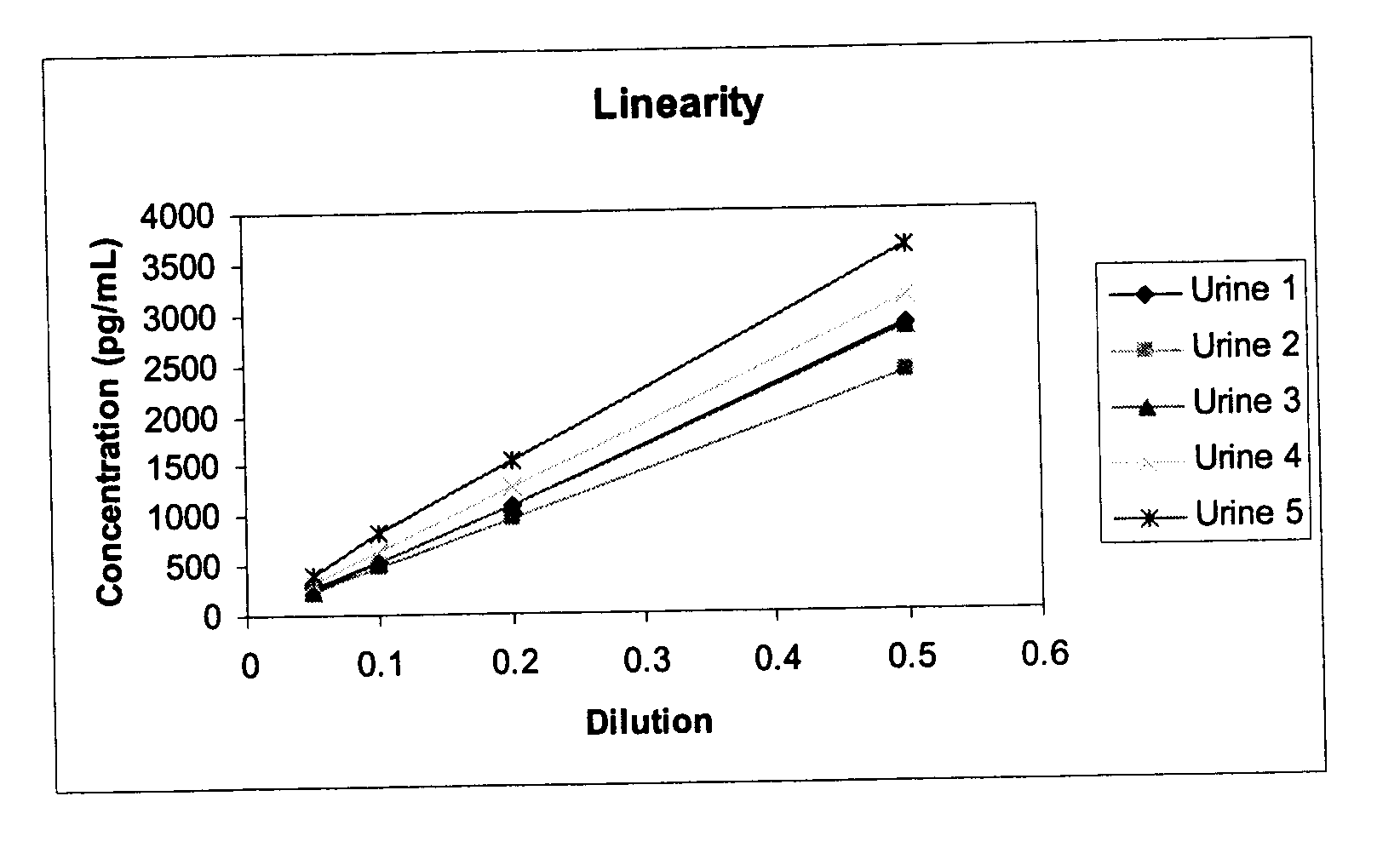
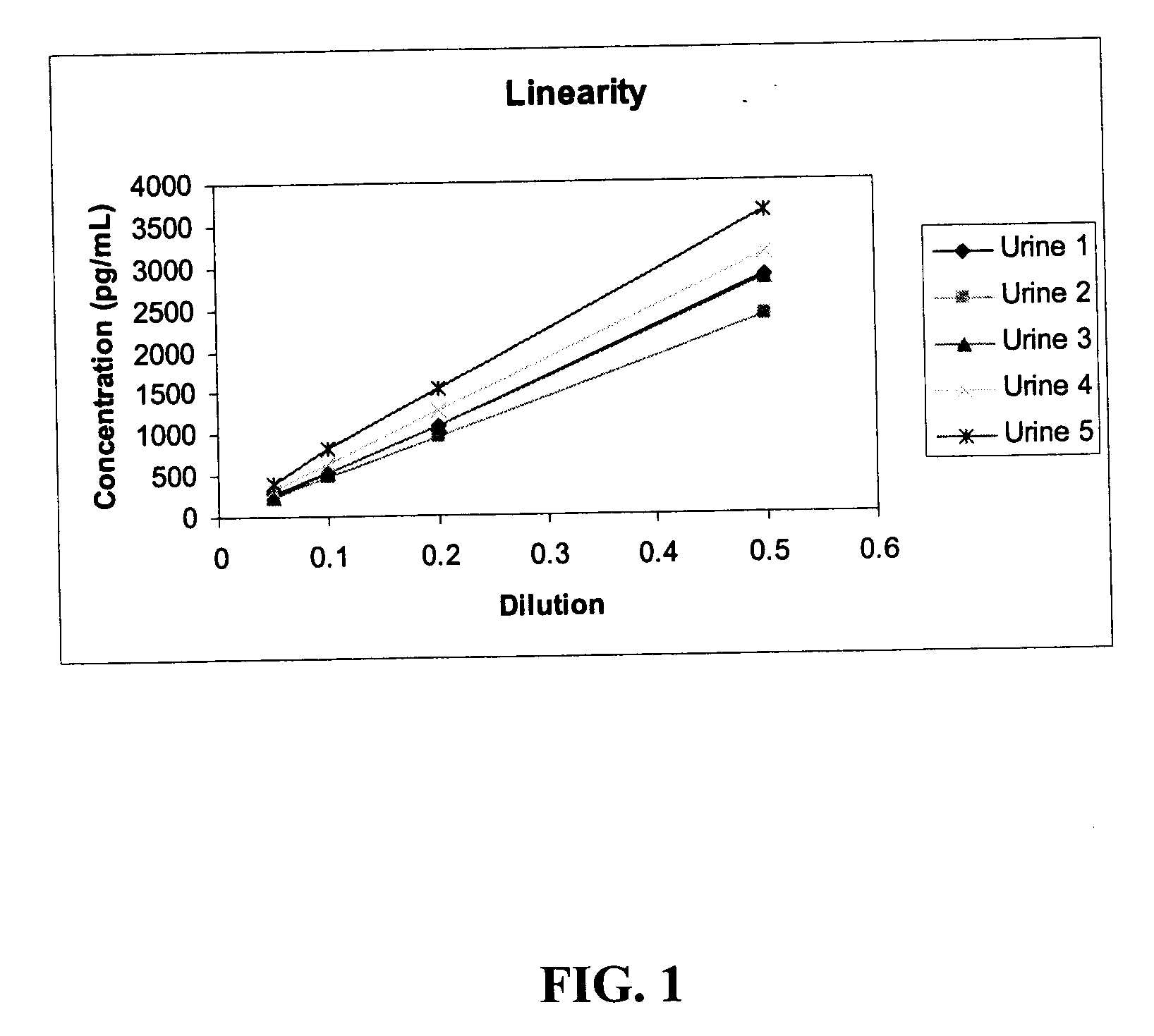
Methods and kits for detection of thromboxane a2 metabolites
Methods, compositions and kits are provided for measuring aspirin's anti-thrombotic effectiveness on a subject. Included are a novel assay for quickly and specifically measuring TxA2 metabolite levels in urine and correlating the levels with aspirin dose in a subject. The methods, compositions and kits utilize a novel anti TxA2 metabolite antibody.
Owner:CORGENIX MEDICAL CORP
Immunofluorescence test strip for fast and quantitatively detecting curative effect of aspirin and preparation method of immunofluorescence test strip
InactiveCN103941007ALow optical densityEasy to operateCompound screeningApoptosis detectionStreptavidinMonoclonal
The invention discloses an immunofluorescence test strip for fast and quantitatively detecting the curative effect of aspirin and a preparation method of the immunofluorescence test strip. The immunofluorescence test strip comprises a support piece as well as a sample gasket, a detection film and a water absorption gasket, which are sequentially overlapped and pasted on the support piece, wherein a conjugate gasket is arranged between the sample gasket and the detection film; a first layer of glass fiber gasket is arranged above one end of the conjugate gasket and a second layer of glass fiber gasket is arranged below one end of the conjugate gasket or only the first layer of glass fiber gasket is arranged above one end of the conjugate gasket or no gasket is arranged; a detection line is arranged on the detection film; the detection line is coated with a 11-dehydro thromboxane B2 (11dhTxB2) monoclonal antibody or polyclonal antibody; a control line is arranged on the other side of the detection line; the control line is coated with an anti-streptavidin (SAV) antibody; the conjugate gasket is coated with fluorescently labeled 11dhTxB2 conjugate. The immunofluorescence test strip is convenient, efficient, simple to operate, accurate in result and suitable for rapid clinical diagnosis.
Owner:RELIA BIOTECH JIANGSU
Hepodxilin analog enantiomers
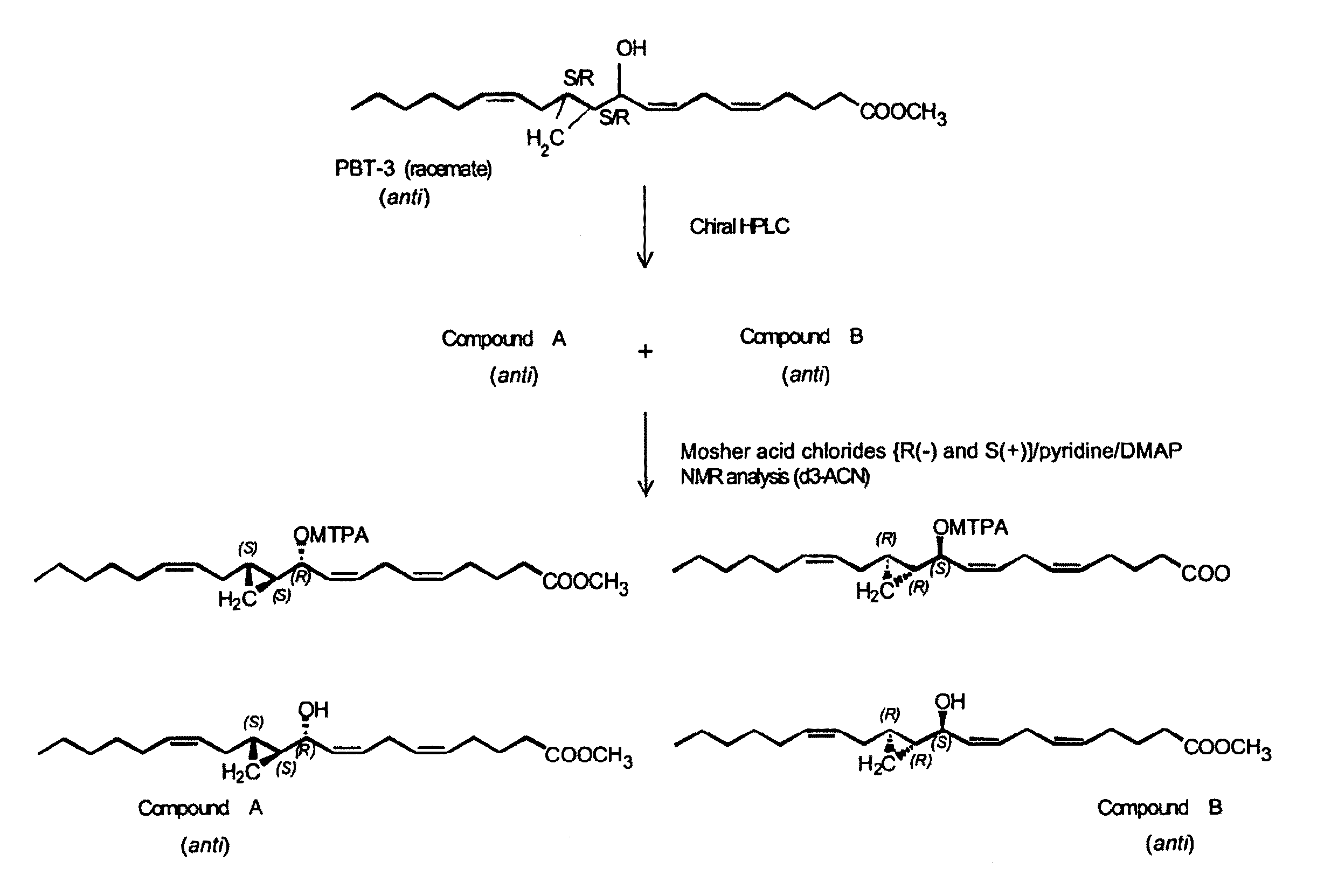
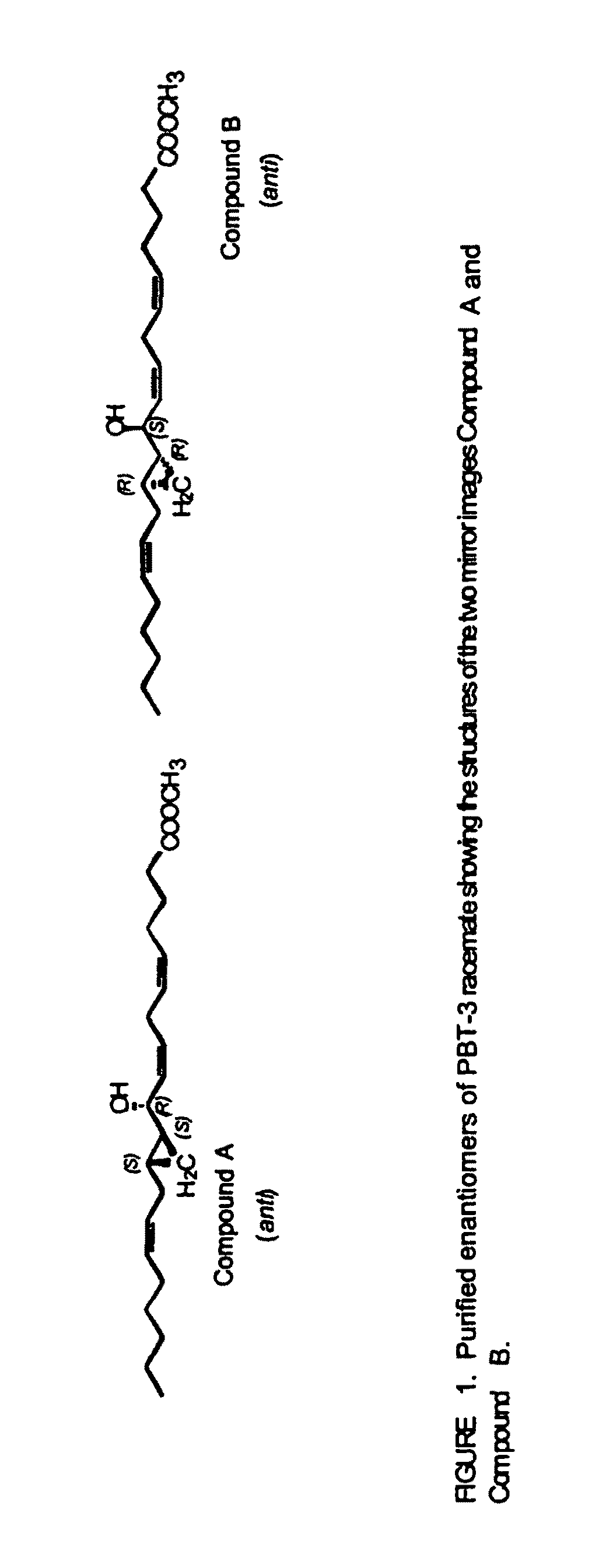
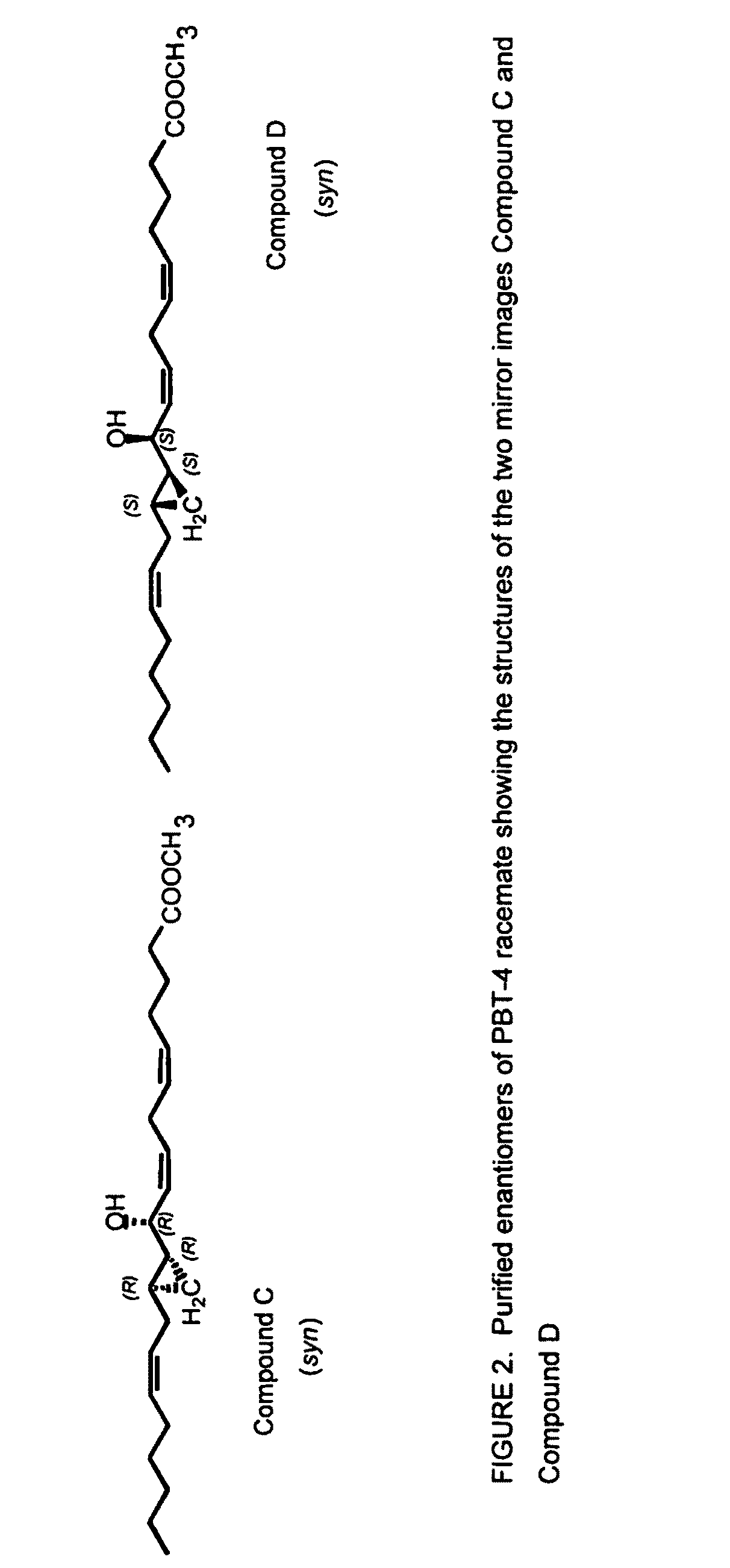
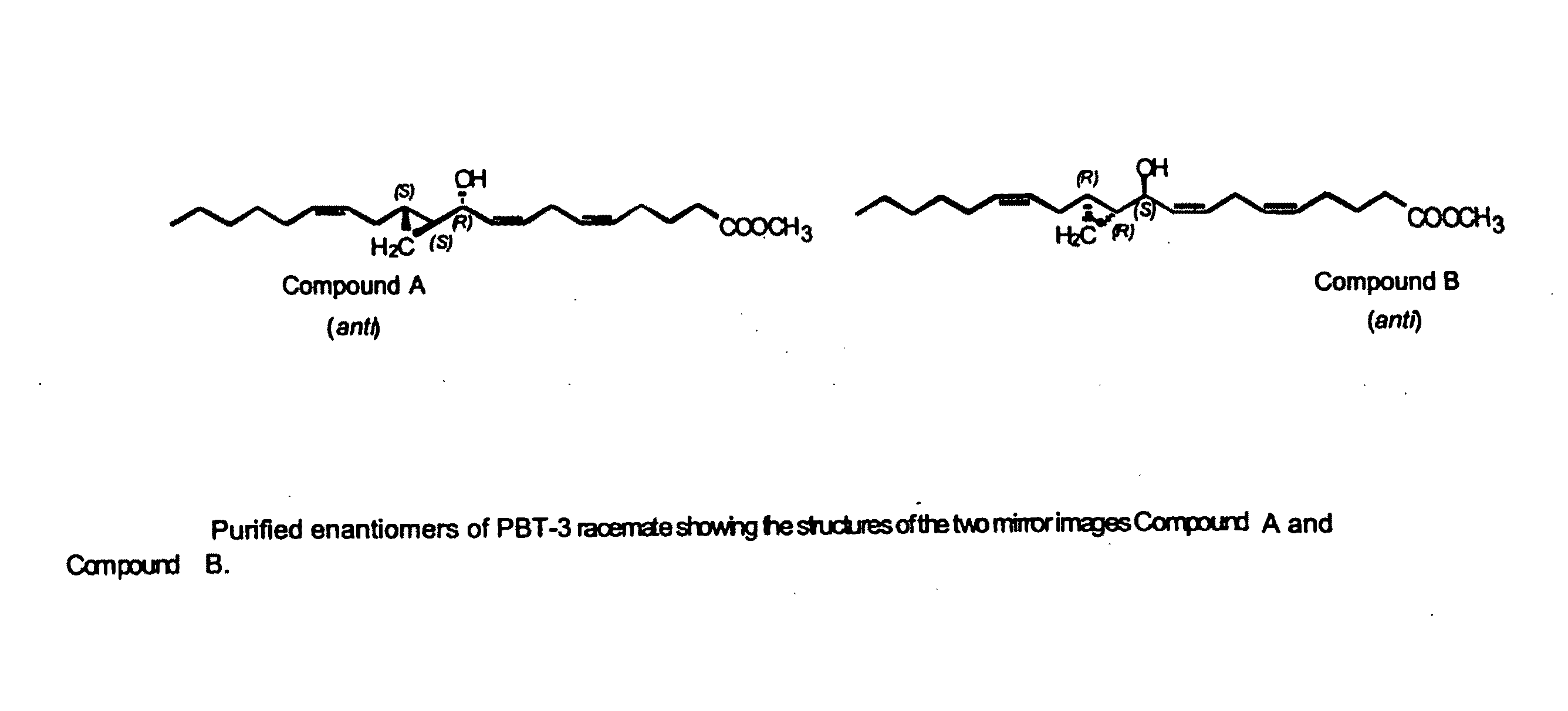
The present invention relates to enantiomeric forms of hepoxilin analogs of Formula I-VIII, pharmaceutical compositions thereof, a method for the separation of said enantiomeric forms of hepoxilin analogs comprising applying said hepoxilin to a chiral phase HPLC column and eluting said hepoxilin with an alkane and alcohol solvent mixture. Said enantiomeric forms of hepoxilin analogs of Formula I-VIII were found to be useful in controlling the biological effects of PPAR mediated transcriptional control for the treatment of diseases such as cancer, thromboxane-mediated diseases and for modulating intracellular calcium concentration.
Owner:CECIL PACE ASCIAK +1
Hepoxilin analog enantiomers
ActiveUS20100210727A1Enhanced chemilluminescenceIncreased cleavageBiocideSenses disorderAlkaneDisease
The present invention relates to enantiomeric forms of hepoxilin analogs of Formula I-VIII, pharmaceutical compositions thereof, a method for the separation of said enantiomeric forms of hepoxilin analogs comprising applying said hepoxilin to a chiral phase HPLC column and eluting said hepoxilin with an alkane and alcohol solvent mixture. Said enantiomeric forms of hepoxilin analogs of Formula I-VIII were found to be useful in controlling the biological effects of PPAR mediated transcriptional control for the treatment of diseases such as cancer, thromboxane-mediated diseases and for modulating intracellular calcium concentration.
Owner:CECIL PACE ASCIAK +1
Method for researching pharmacodynamic relationship of various medicinal components of compound thromboxane preparation
InactiveCN107742058ASubstantively innovativeGood practice valueMolecular designSpecial data processing applicationsUniform designMedicine
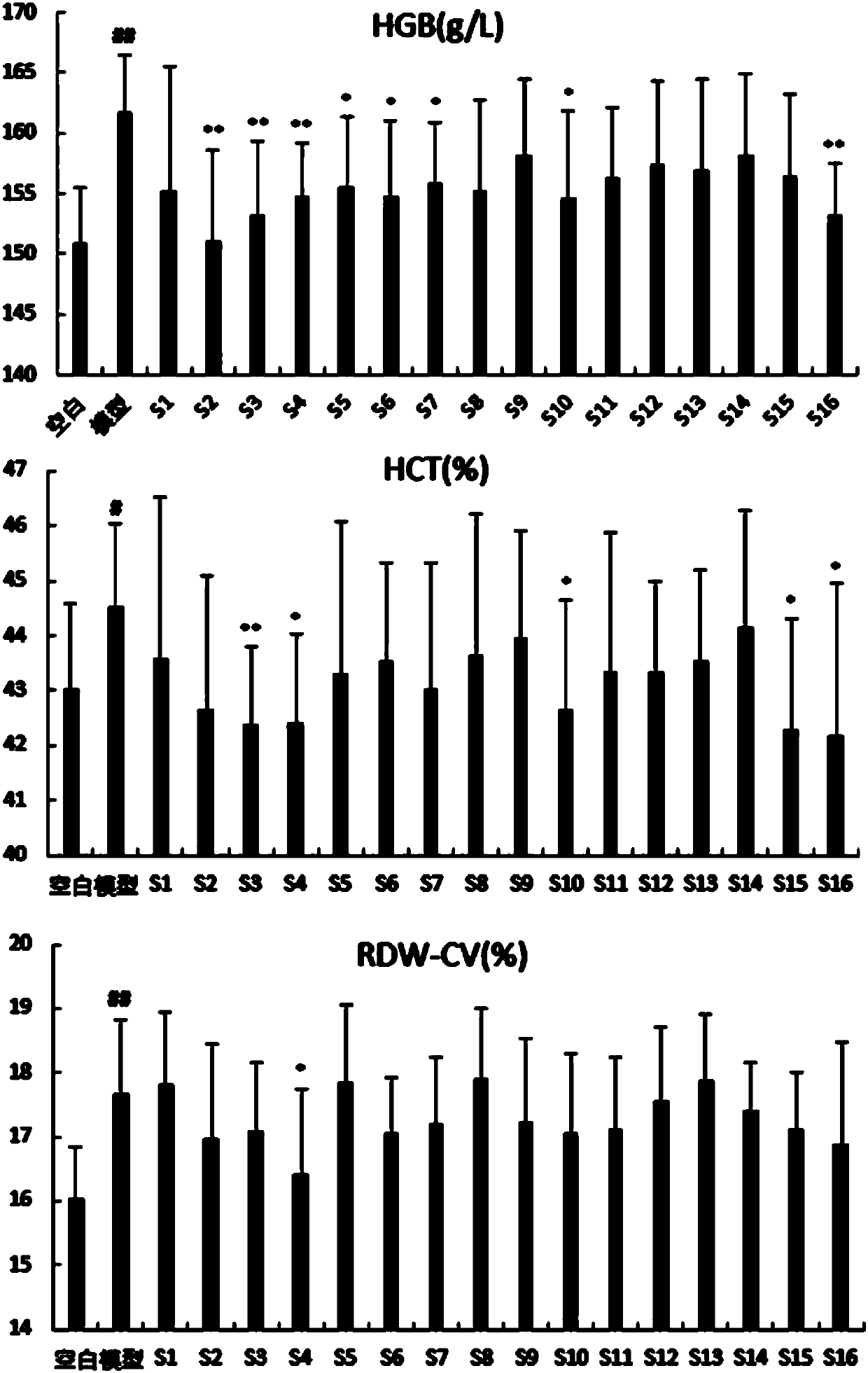
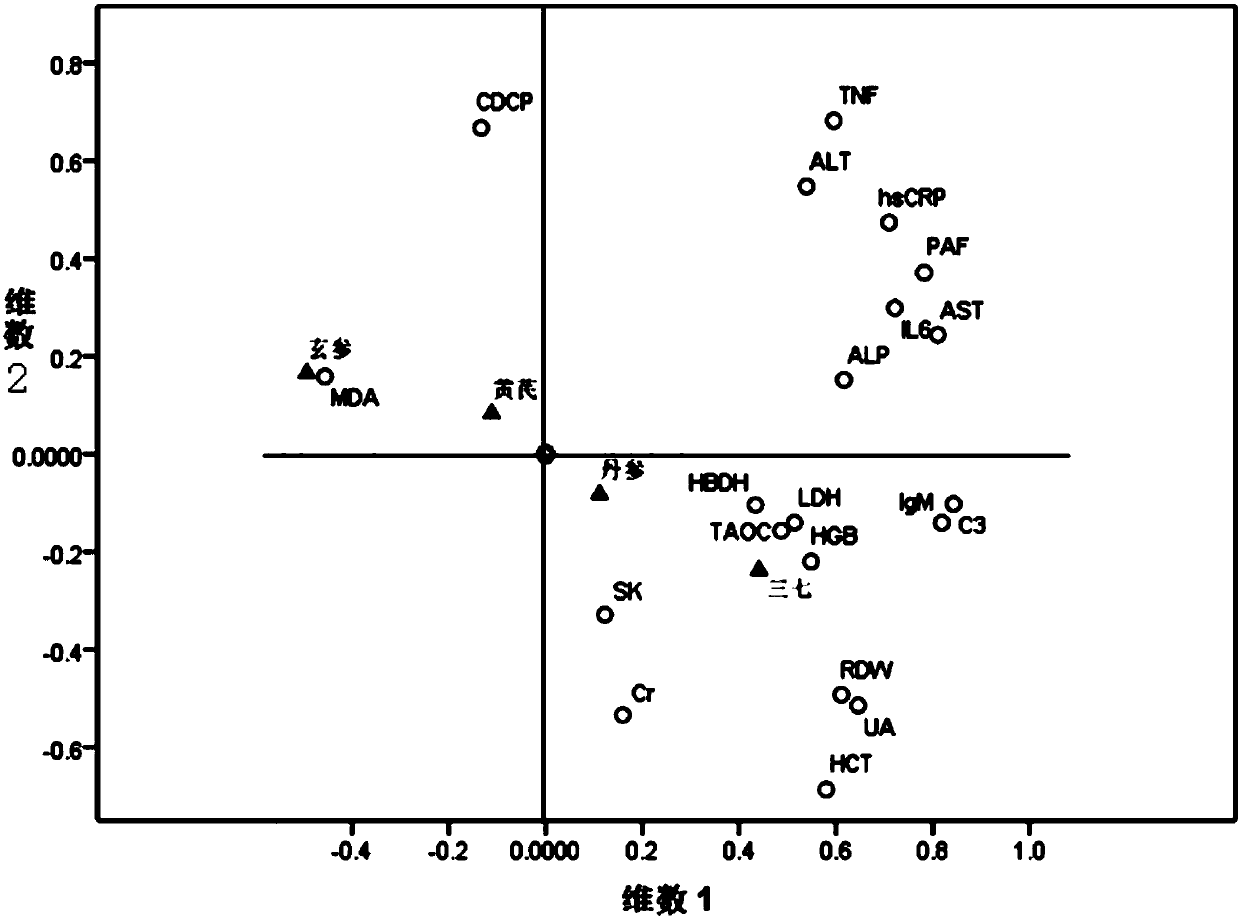
The present invention discloses a method for researching the pharmacodynamic relationship of various medicinal components of a compound thromboxane preparation. In the method, the mass percentage of each herbal medicine in the formula of the compound thromboxane preparation is taken as a variable, a uniform design method is used to prepare multiple difference samples with different proportions ofmedicinal materials, and at the same time, multiple missing samples of prescription medicinal materials is prepared; through pharmacodynamics research, the pharmacodynamic data of each difference sample and missing sample of the medicinal materials are obtained; and a variety of statistical methods are used to analyze the relationship between medicinal materials and efficacy in multiple samples, and the contribution, primary and secondary effects and interactions of each medicinal material are determined. The present invention discloses for the first time a method for researching the contribution, primary and secondary effects and interactions of each medicinal material in the compound thromboxane preparation, provides a basis for clarifying the compatibility rule of the scientific constituents and screening the optimal proportion of the constituents, and provides more scientific and more complete modern scientific data support for the clinical application of the compound thromboxane preparation.
Owner:SUN YAT SEN UNIV
Von willebrand factor specific binding agents and uses thereof
InactiveUS20120321640A1Prevent thrombosisReduce brain damageImmunoglobulins against blood coagulation factorsAntibody mimetics/scaffoldsDiseaseThrombus
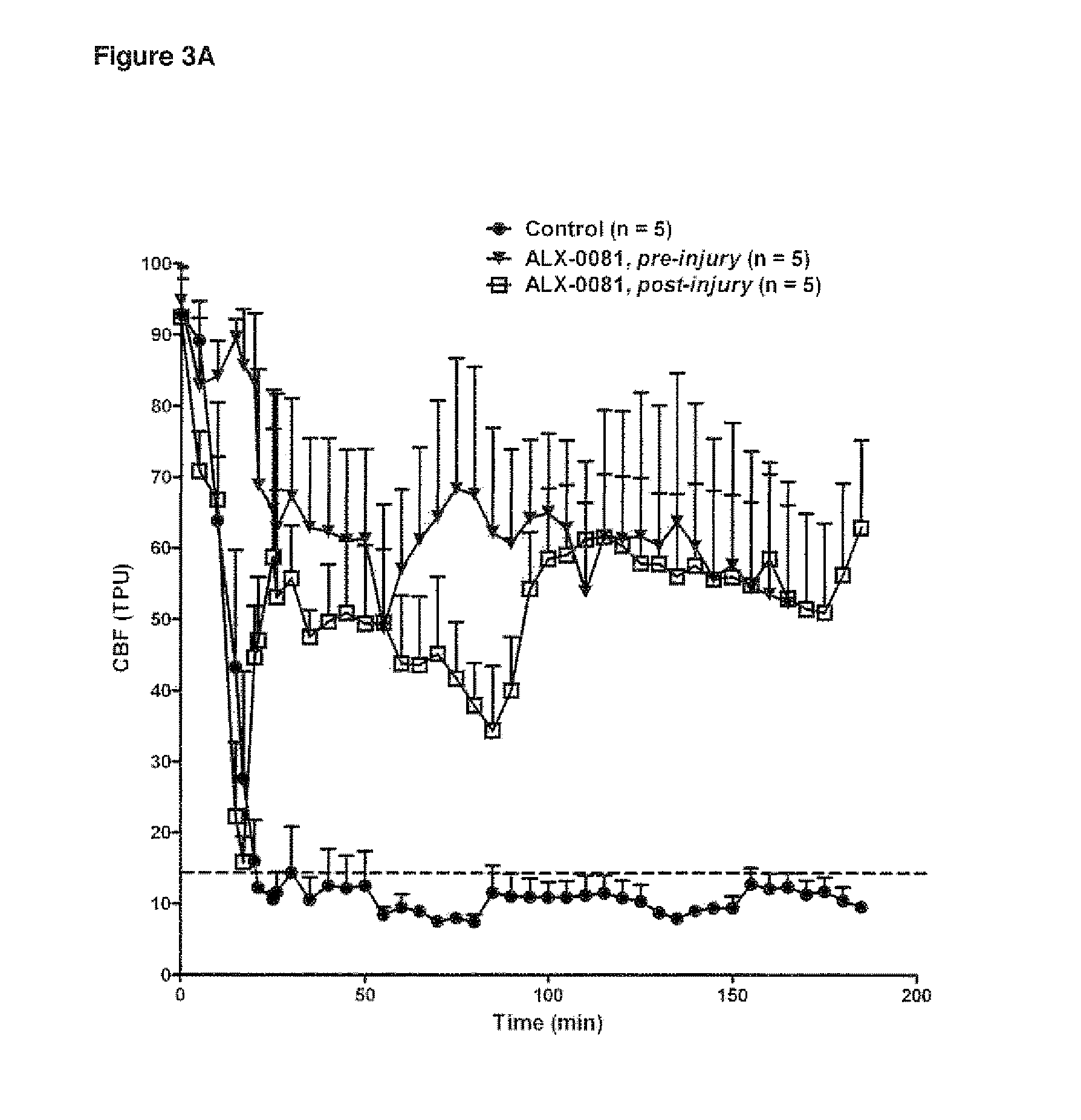
The invention provides new uses, compositions and methods of administration for specific binding agents to von Wiliebrand Factor (vWF) in patients with thromboembolic disorders and in particular new combined uses with thrombolytic agents such as tissue plasminogen activator in patients with thromboembolic disorders such as e.g. ischemic stroke. Furthermore, a new group of vWF binding agents and an improved Middle Cerebral Artery Thrombosis Model in guinea pigs to study the effects of stroke such as ischemia (oxygen and glucose depriviation) and hemorrhage (bleeding), in particular hemorrhage, are provided.
Owner:ABLYNX NV
Process for Obtaining Recombinant Prothrombin Activating Protease (Rlopap) in Monomeric form; the Recombinant Prothrombin Activating Protease (Rlopap) as Well as its Amino Acid Sequence; the Use of this Protease as a Defibrinogenase
InactiveUS20080267944A1Sugar derivativesPeptide/protein ingredientsDysprothrombinemiaADAMTS Proteins
This invention refers to the process for obtaining the recombinant prothrombin activating protease (rLopap) in monomeric form, the recombinant prothrombin activating protease (rLopap), as well as its amino acid sequence. In addition to that, this invention also refers to the use of this protease for depleting the blood fibrinogen, and serve as diagnosis kit for dysprothrombinemias. This invention describes the obtainence in recombinant form and the characterization of a prothrombin activator protease of 21 kDa, named rLopap (Lonomia obliqua prothrombin activator protease), with serineproteases characteristics however it shows sequence of conserved amino acids as in a lipocalin family. The protein presents pro-coagulating activity, depleting blood fibrinogen and prolonging the coagulation time of human blood / plasma. The obtainence of rLopap in its recombinant form and showing adequate activity for allowing clinical Pharmacology essays is presented in this invention.
Owner:BIOLAB SANUS FARMACEUTICA LTD +1
Methods and kits for detection of thromboxane A2 metabolites
Methods, compositions and kits are provided for measuring aspirin's anti-thrombotic effectiveness on a subject. Included are a novel assay for quickly and specifically measuring TxA2 metabolite levels in urine and correlating the levels with aspirin dose in a subject. The methods, compositions and kits utilize a novel anti TxA2 metabolite antibody.
Owner:CORGENIX MEDICAL CORP
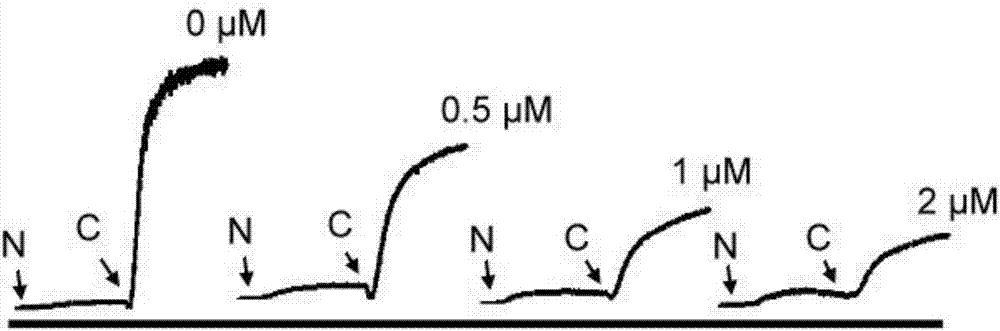
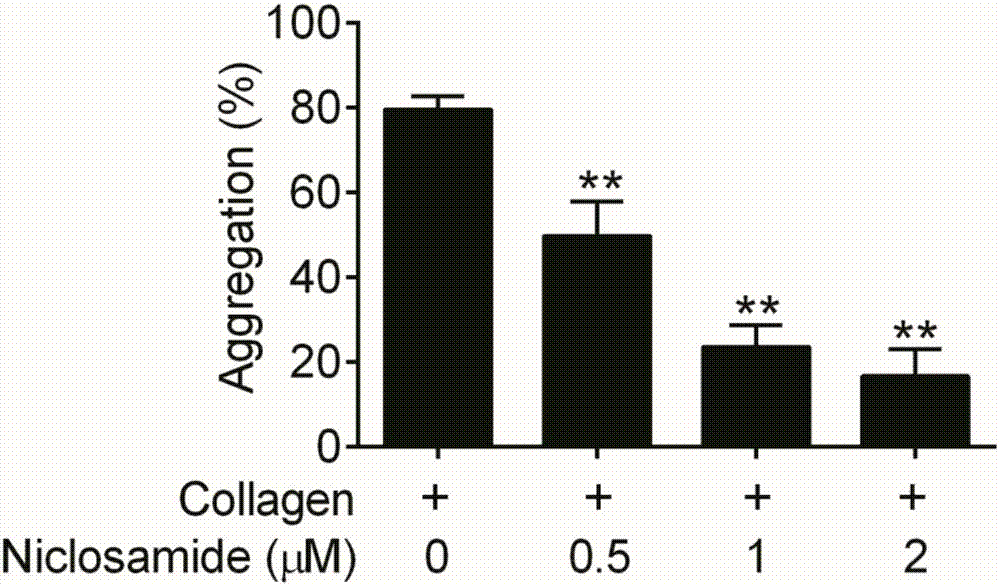
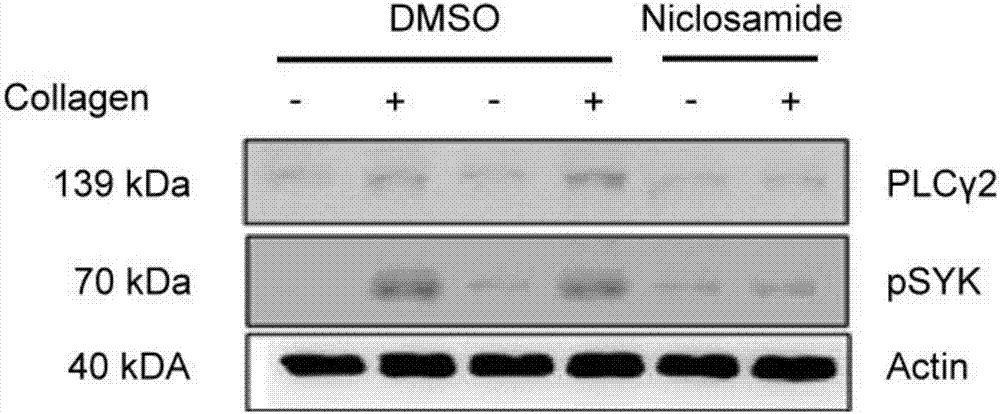
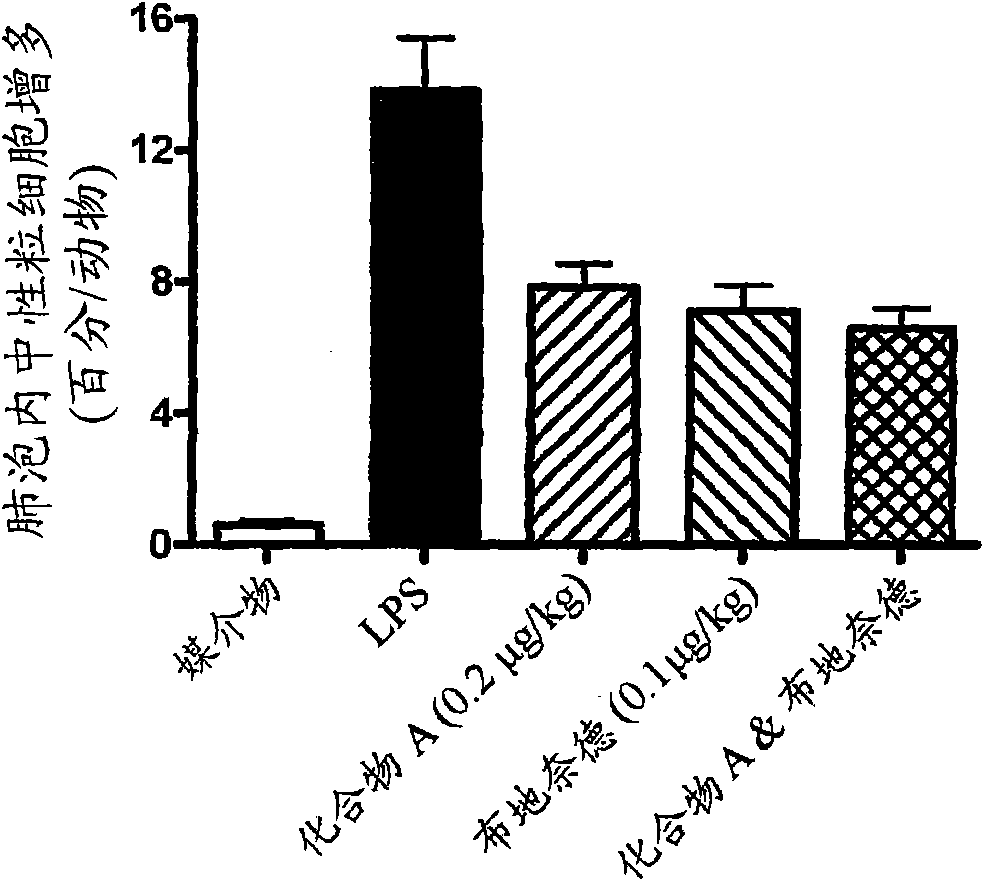
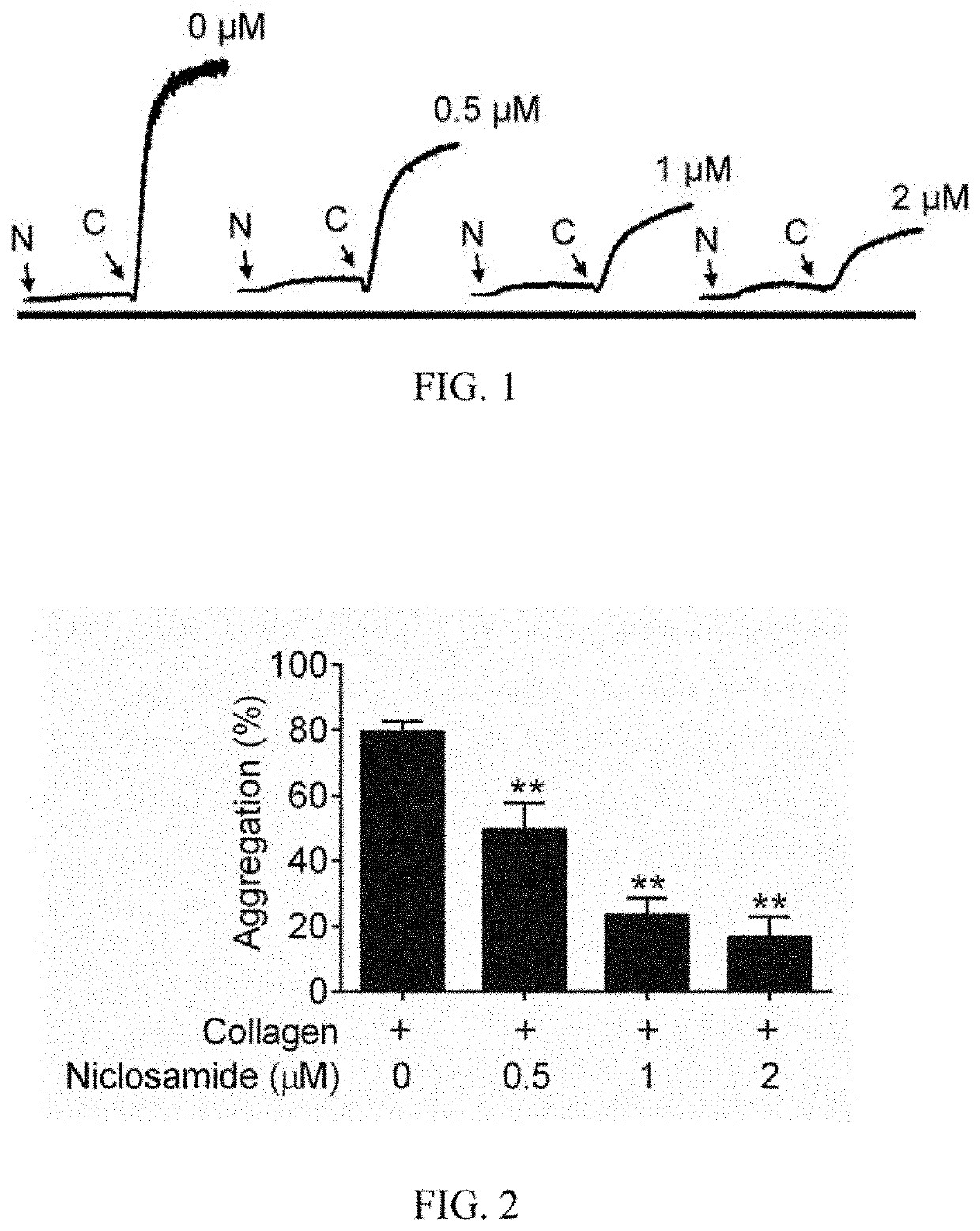
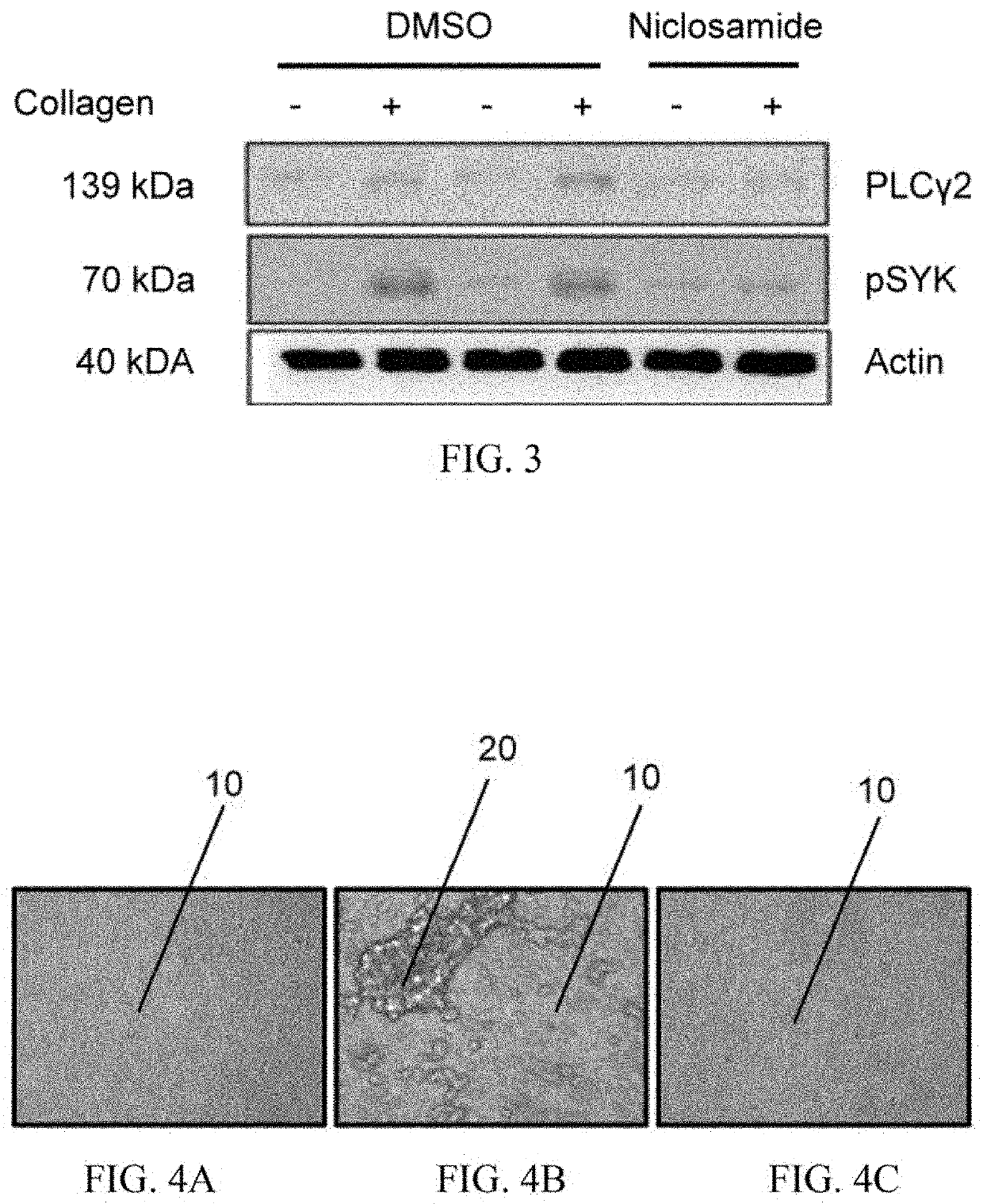
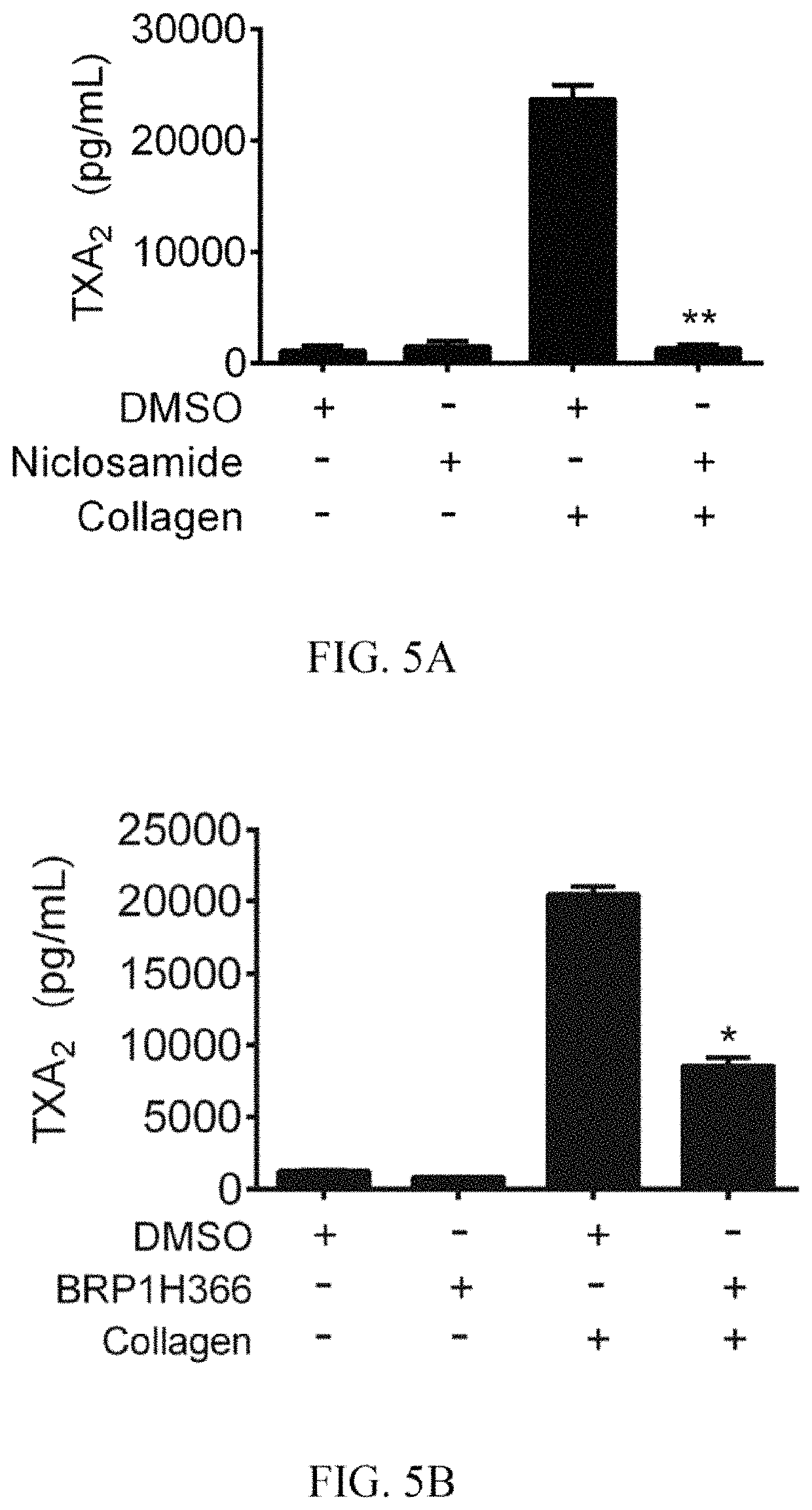
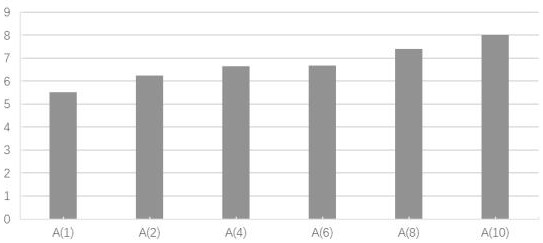
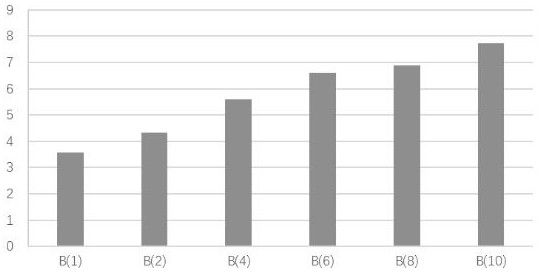
Niclosamide and applicaiton of niclosamide derivatives
InactiveCN107951895AOrganic active ingredientsHydrolysed protein ingredientsDiseasePharmaceutical drug
The invention discloses niclosamide and an application of niclosamide derivatives. The niclosamide and niclosamide derivatives are used for the manufacture of medicaments for suppressing platelet aggregation or preventing thrombosis-related diseases. The niclosamide and niclosamide derivatives in the medicaments inhibit the production of thromboxane A2, therefore suppress platelet aggregation andprevent thrombosis-related diseases.
Owner:CHANG GUNG UNIVERSITY
Combinations of beta- 2 -adrenoceptor agonistic benzothiazolone
The invention provides a pharmaceutical product comprising a first active ingredient which is N-[2-(Diethylamino)ethyl]-N-(2-{[2-(4-hydroxy-2-oxo-2,3-dihydro-1,3-benzothiazol-7-yl)ethyl]amino}ethyl)-3-[2-(1-naphthyl)ethoxy]propan amide or a salt thereof, and a second active ingredient selected from: a non-steroidal Glucocorticoid Receptor (GR Receptor) Agonist; an antioxidant; a CCR1 antagonist; achemokine antagonist (not CCR1); a corticosteroid; a CRTh2 antagonist; a DP1 antagonist; an Histone Deacetylase Inducer; an IKK2 inhibitor; a COX inhibitor; a lipoxygenase inhibitor; a leukotriene receptor antagonist; an MPO inhibitor; a muscarinic antagonist which is Aclidinium bromide, Glycopyrrolate, Oxitropium bromide, Pirenzepine, telenzepine, Tiotropium bromide,3(R)-(2-hydroxy-2,2-dithien-2-ylacetoxy)-1-(3-phenoxypropyl)-1-azoniabicyclo[2.2.2]octane bromide,3(R)-1-phenethyl-3-(9H-xanthene-9-carbonyloxy)-1-azoniabicyclo[2.2.2]octane bromide or (3R)-3-[(2S)-2-cyclopentyl-2-hydroxy-2-thien-2-ylacetoxy]-1-(2-phenoxyethyl)-1-azoniabicyclo[2.2.2]actane bromide; a p38 inhibitor; a PDE inhibitor; a PPARy agonist; a protease inhibitor; a Statin; a thromboxane antagonist; a vasodilator; or, an ENAC blocker (Epithelial Sodium-channel blocker); and its use in the treatment of respiratory disease.
Owner:ASTRAZENECA AB
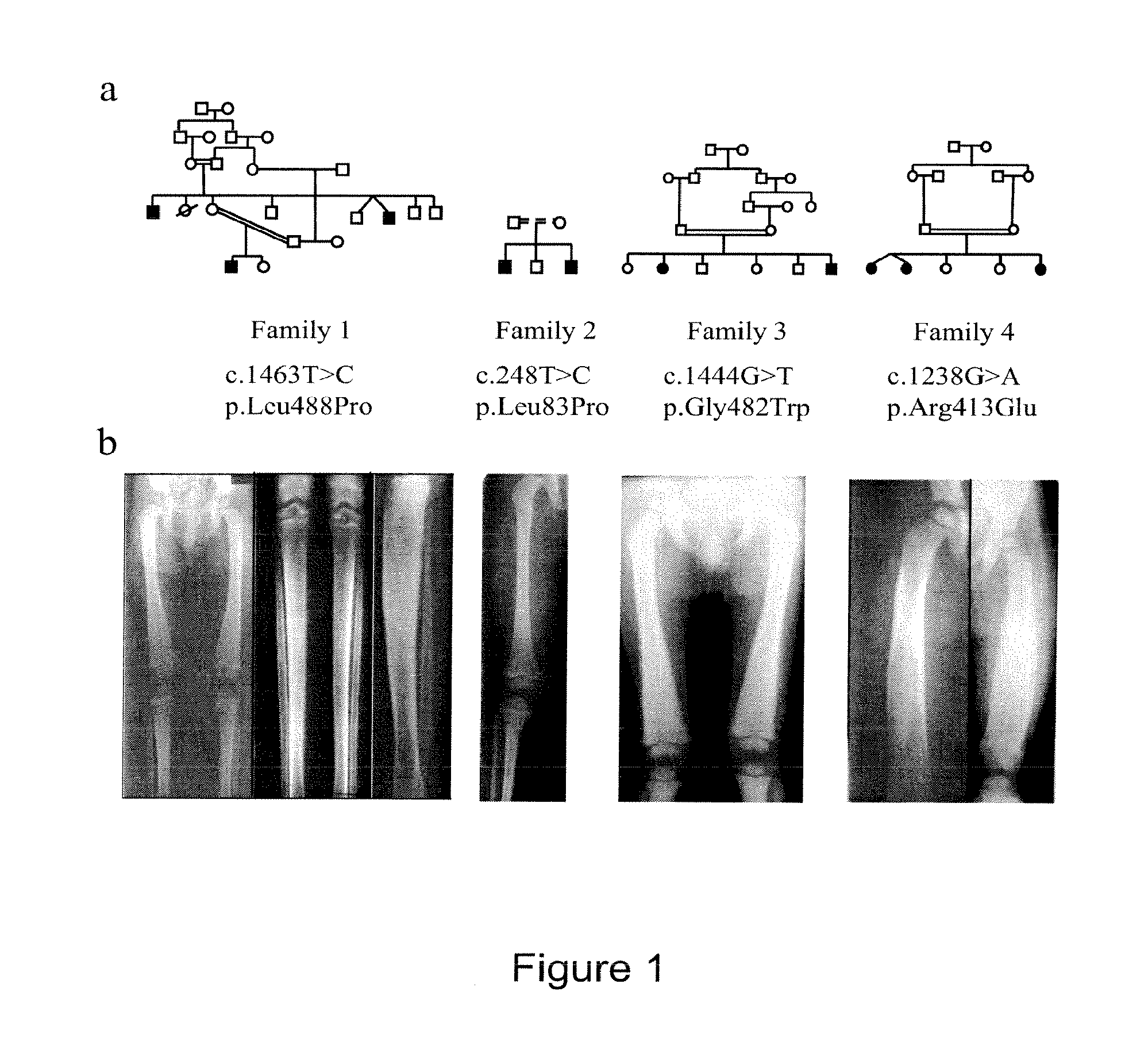
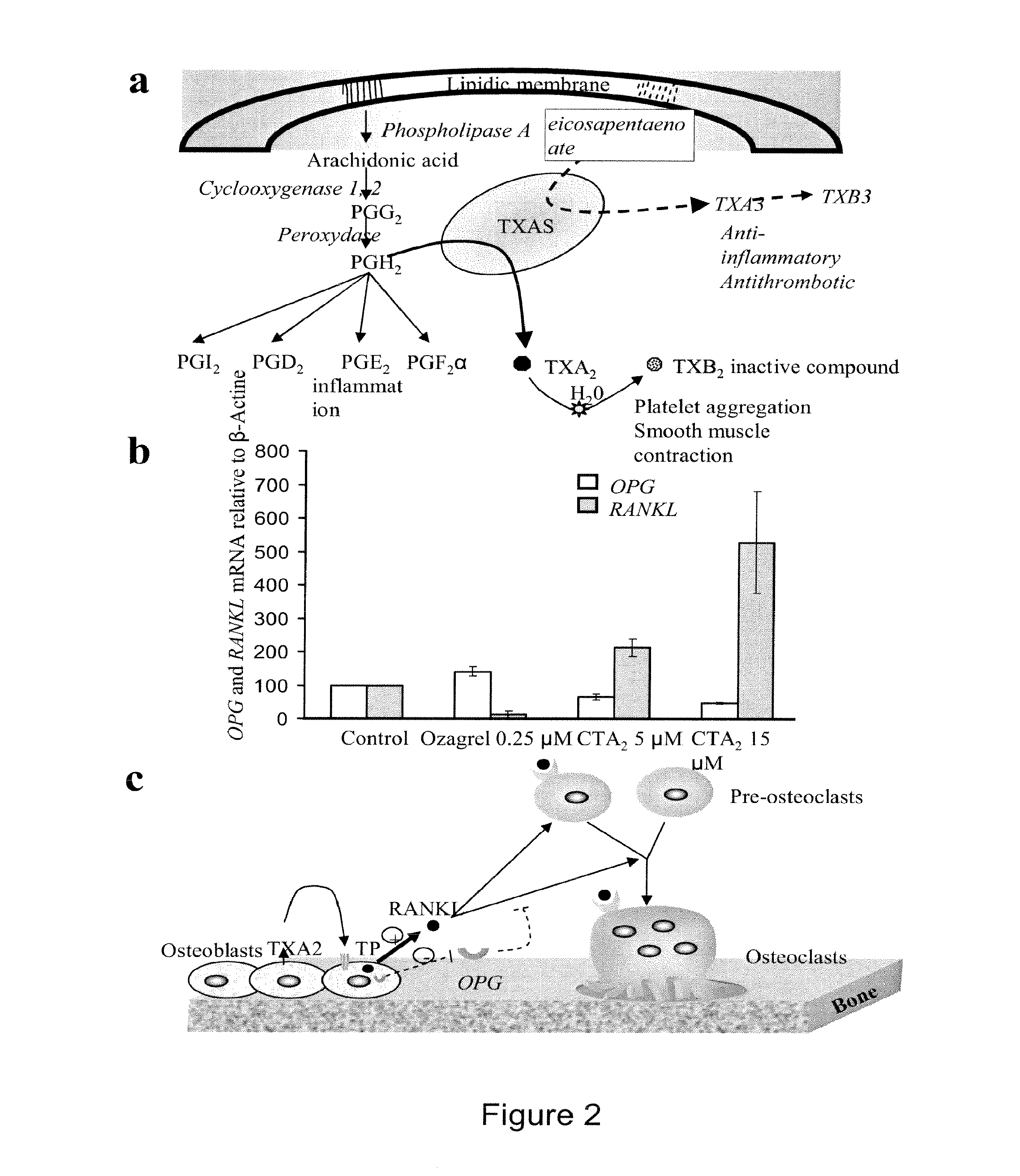
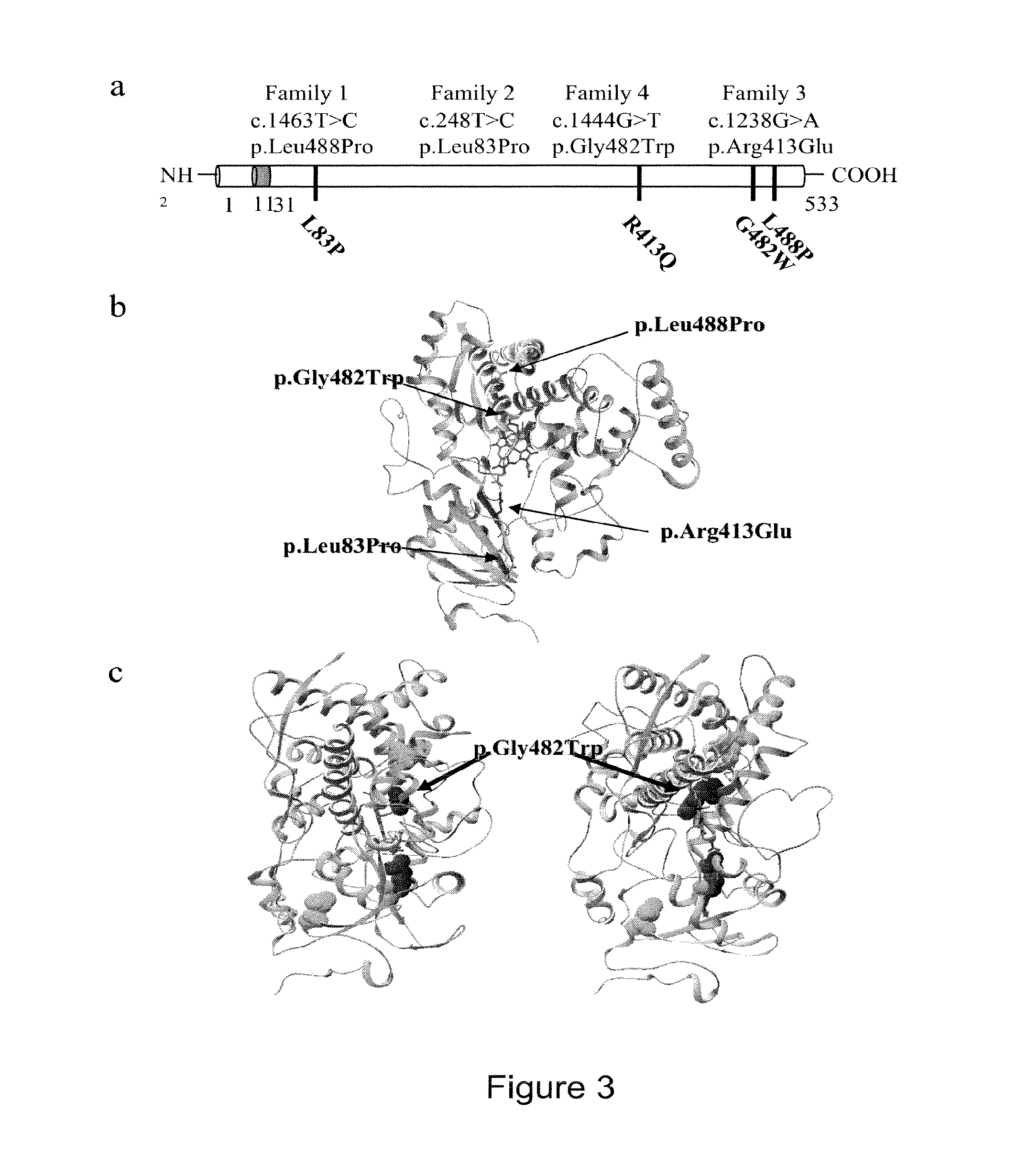
Methods for the treatment and diagnosis of bone mineral density related diseases
ActiveUS20150104437A1Difficult to solveAvoid side effectsBiocideOrganic active ingredientsGhosal hematodiaphyseal dysplasiaDisease
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +2
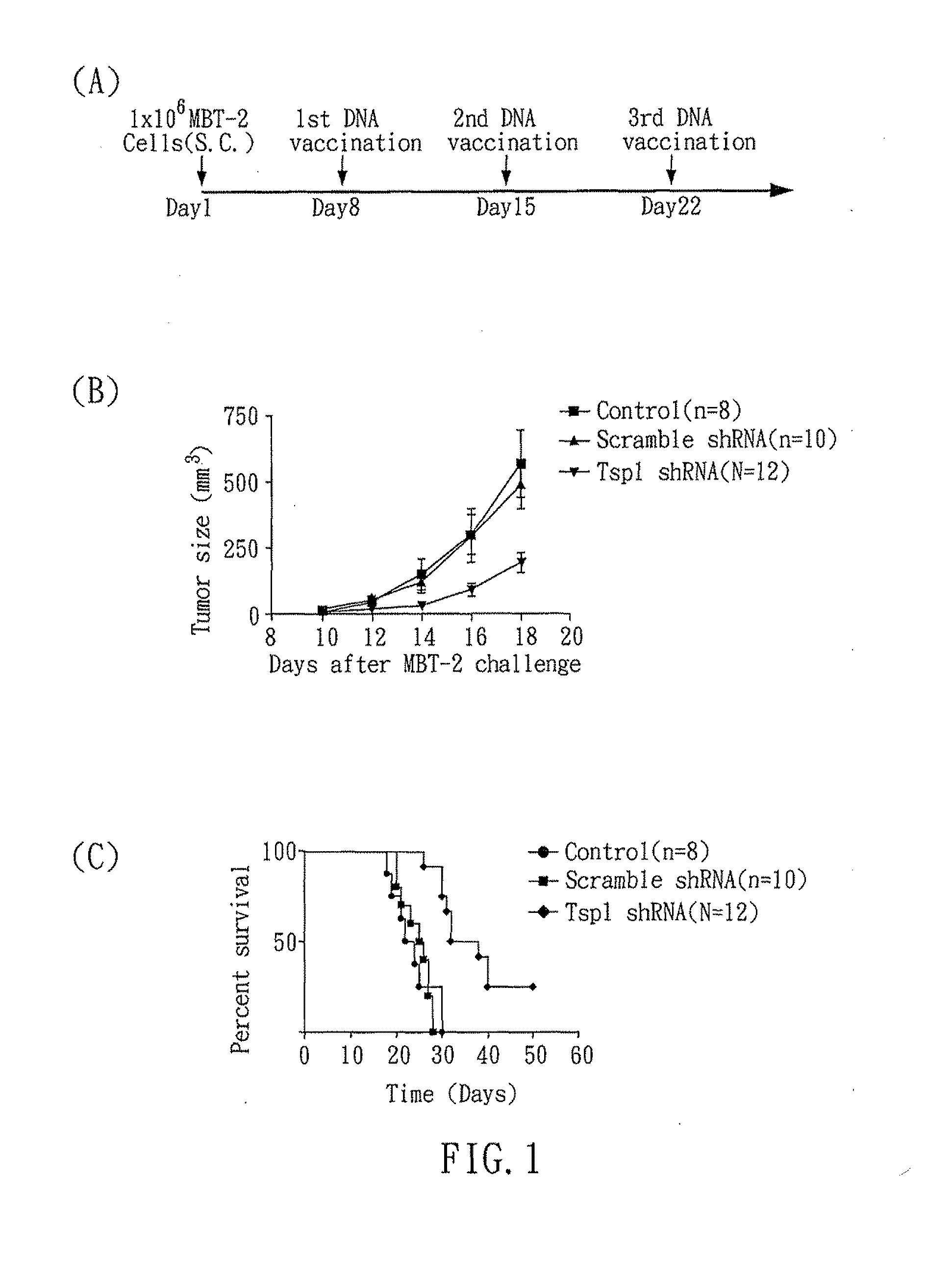
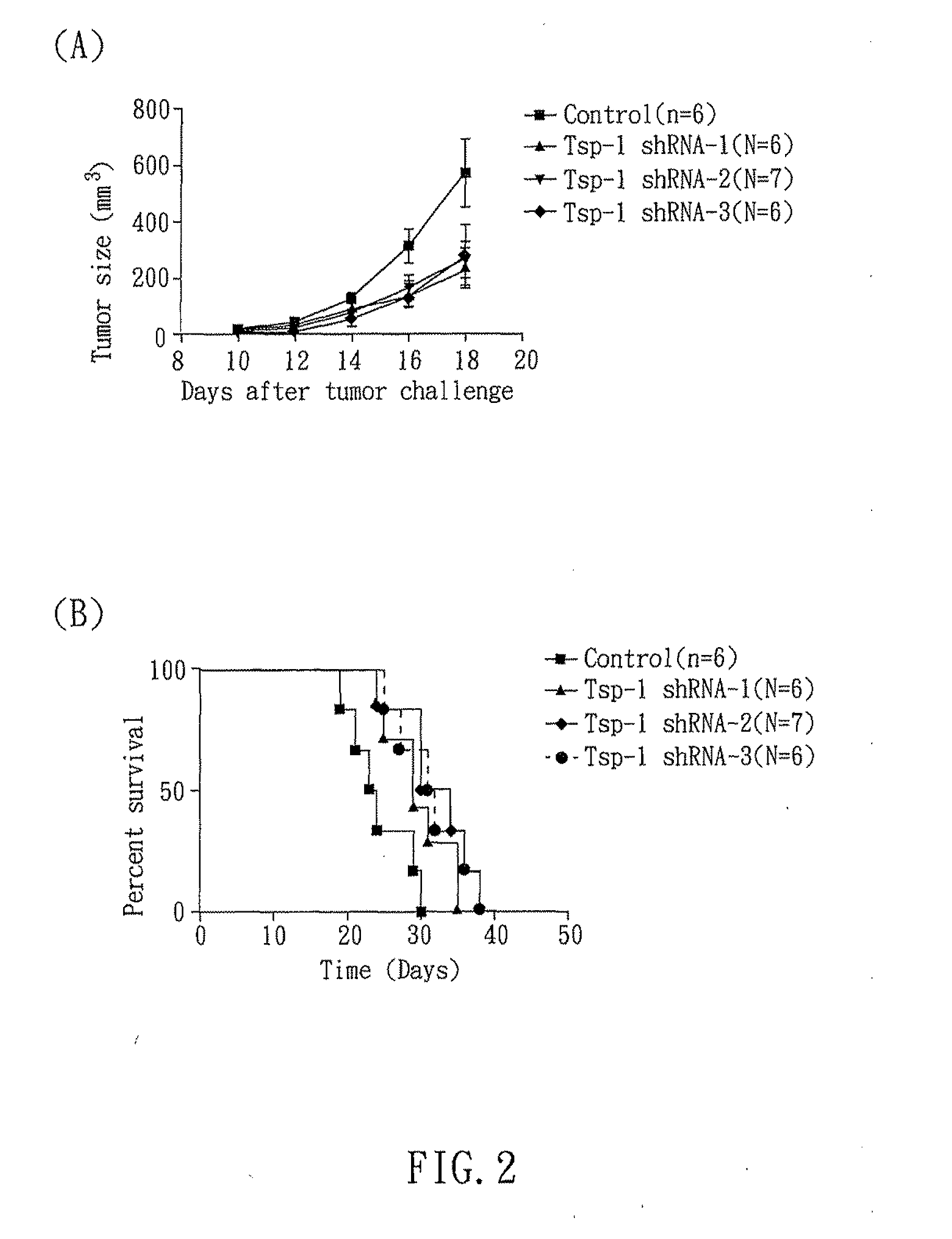
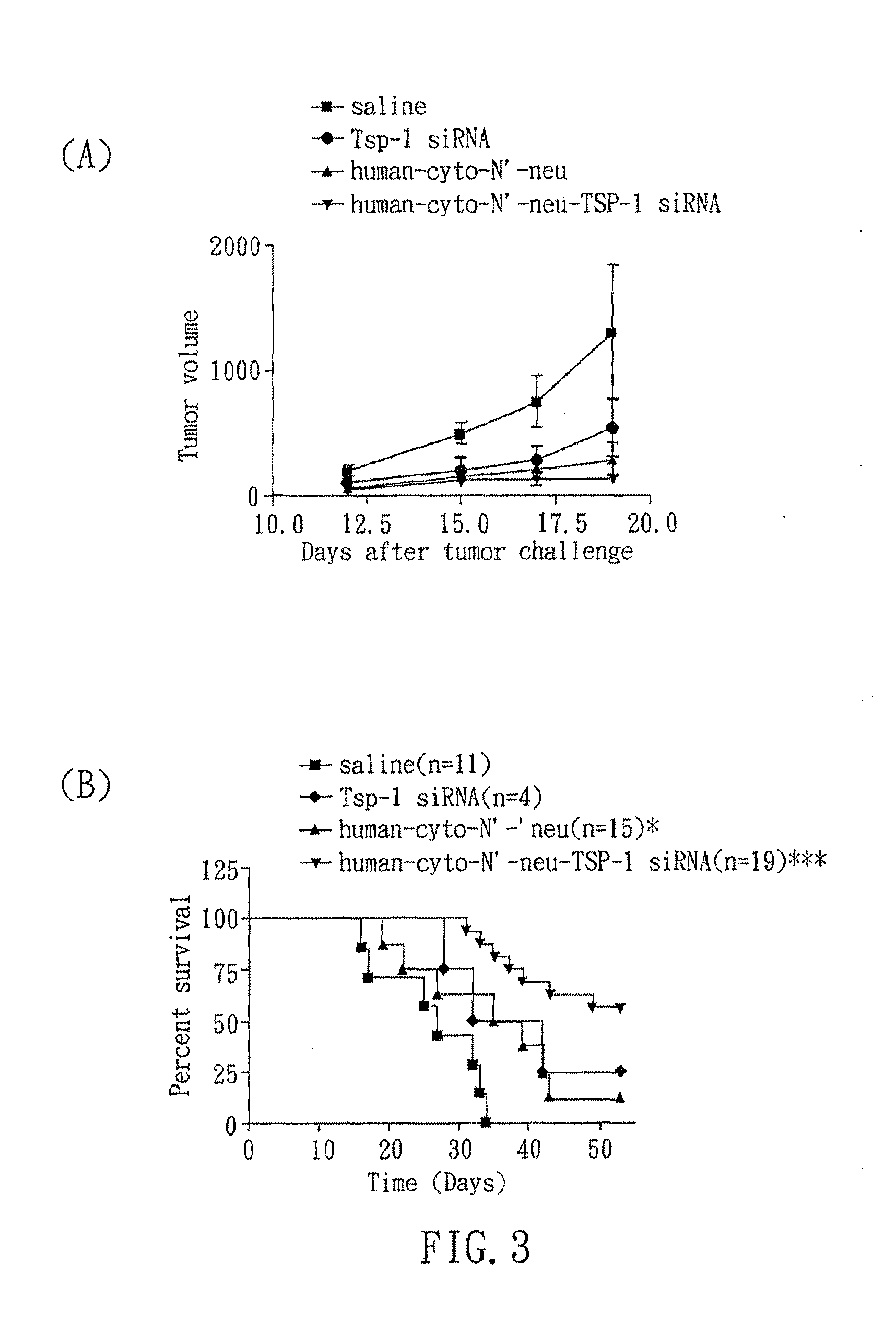
RNAi COMPOUND TARGETED TO THROMBOSPONDIN-1 AND APPLICATIONS THEREOF
InactiveUS20110166199A1Inhibit expressionStrong specificityOrganic active ingredientsSugar derivativesTumor progressionEukaryotic plasmids
The present invention relates to an RNAi compound and an expression plasmid for inhibiting expression of Thrombospondin-1, which comprises a target sequence selected from Thrombospondin-1 gene. The present invention also related to a pharmaceutical composition comprising the RNAi compound and applications thereof. The RNAi compound can reduce the expression of Thrombospondin-1 to activate immune responses. In addition, the present invention also disclosed that an RNAi compound targeted to Thrombospondin-1 gene can delay tumor progression.
Owner:NAT CHENG KUNG UNIV
Detection kit for detecting metabolic capability of aspirin and preparation method of detection kit
ActiveCN114002433AImprove capture efficiencyHigh detection sensitivityMaterial analysis by observing effect on chemical indicatorDisease diagnosisAnti plateletImmuno detection
The invention discloses a detection kit for detecting the metabolic capability of aspirin and a preparation method of the detection kit, and the kit comprises an elisa plate coated with a monoclonal antibody of 11-dehydrothromboxane B2, a 11-dehydrothromboxane B2 competitive product, a chromogenic substrate, a stop buffer and a 11-dehydrothromboxane B2 standard solution, wherein the monoclonal antibody of 11-dehydrothromboxane B2 is a mouse monoclonal antibody, the sequence of a light chain variable region of the mouse monoclonal antibody is as shown in SEQ ID NO.2 in a sequence table, and the sequence of a heavy chain variable region of the mouse monoclonal antibody is as shown in SEQ ID NO.4 in the sequence table. The enzyme-linked immunosorbent assay kit and the method thereof have the characteristics of accuracy, high sensitivity and the like for detecting aspirin metabolism so as to judge whether a patient has aspirin drug resistance or resistance, and are not easily influenced by biochemical indexes such as creatinine and the like; and the problem of failure or poor effect of anti-platelet aggregation treatment caused by aspirin resistance of clinical patients can be greatly reduced.
Owner:湖南菲思特精准医疗科技有限公司
A strain of Lactobacillus plantarum ua149 and its application
ActiveCN110055199BLower blood uric acid levelsReduce synthesisBacteriaSkeletal disorderBiotechnologyInflammatory factors
A strain of Lactobacillus plantarum UA149 and its application belong to the field of functional food microorganisms. A strain of Lactobacillus plantarum (L. plantarum) UA149 of the present invention has been preserved in the China Center for Type Culture Collection on November 29, 2018, with the preservation number CCTCC NO: M2018842. The bacterial strain can be used in the preparation of products with uric acid-lowering function or anti-gout function. The invention takes the lactic acid bacteria isolated and identified from the surface of succulent leaves as the research object, and a new strain of the lactic acid bacteria is screened out through a large number of experiments. Hyperuricemia model rats were established by using potassium oxonate combined with fructose water, and intragastric administration of Lactobacillus plantarum UA149 strain for 14 days could significantly reduce blood uric acid levels; during gout attacks, neutrophil-mediated inflammation could be reduced. The release of sex factor thromboxane and leukotrienes prevents the influx of neutrophils into the joints and reduces redness, pain, heat and other symptoms.
Owner:JILIN MINGZHIYUAN BIOTECH
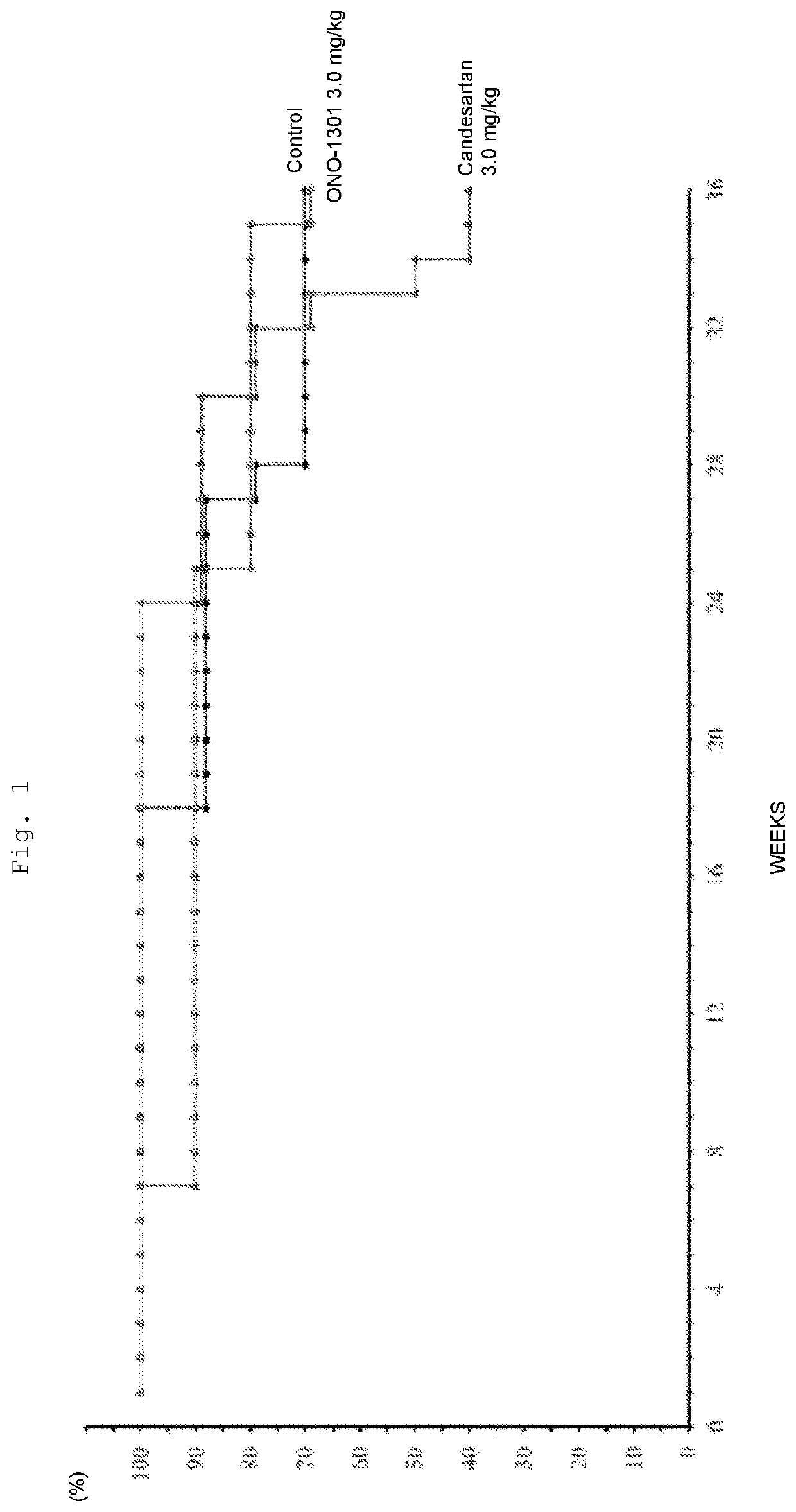
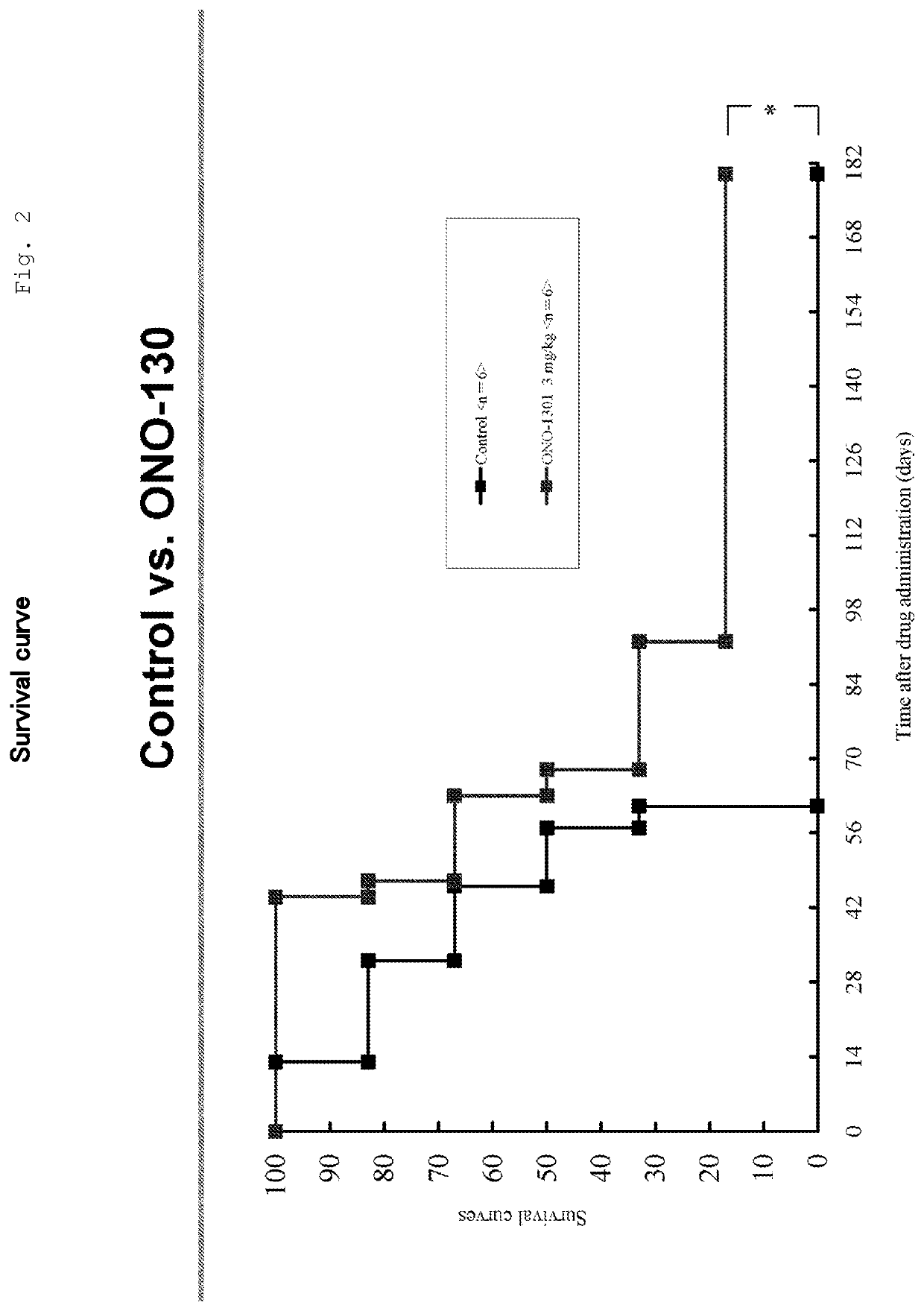
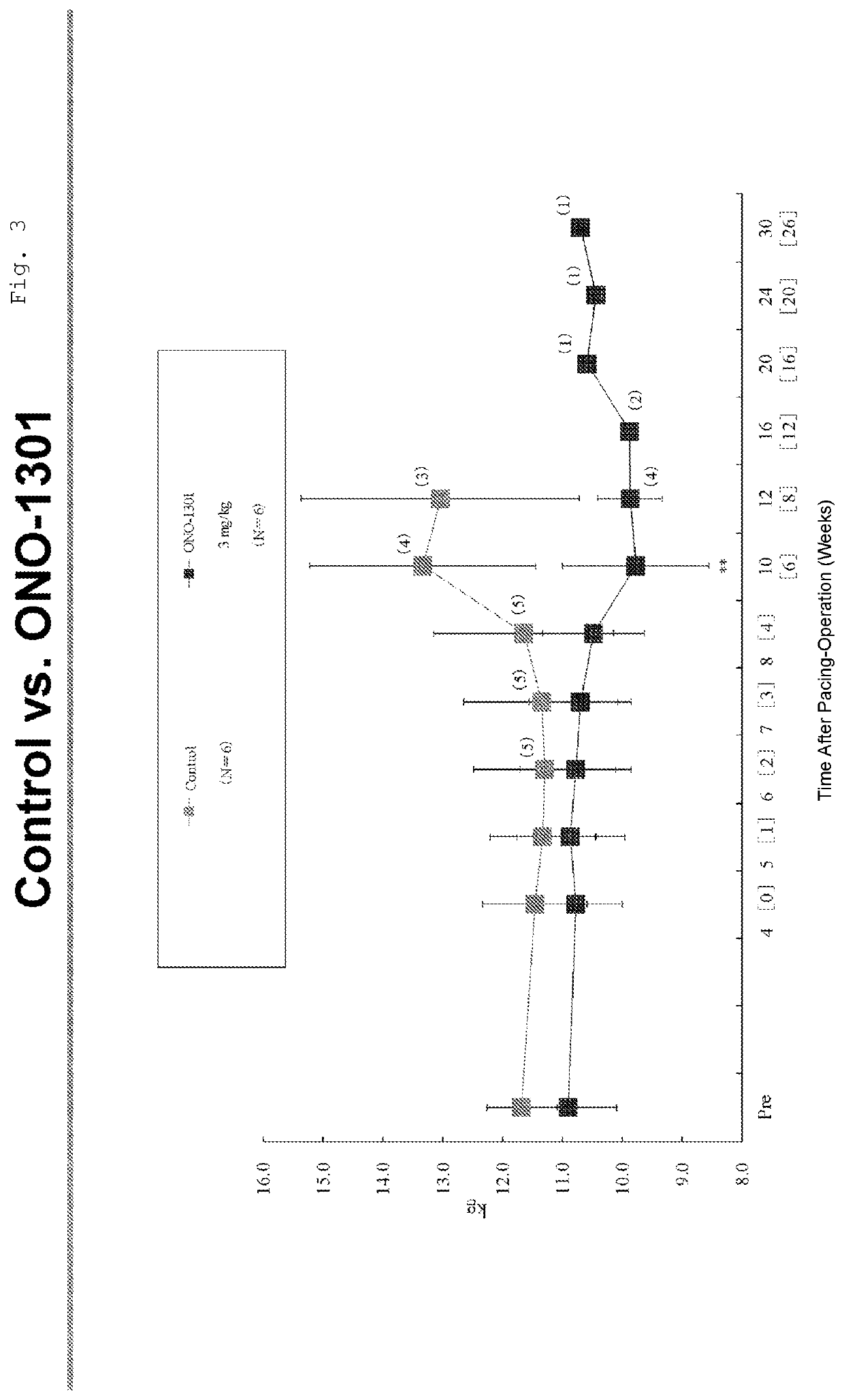
Medicinal Composition for Treating Intractable Heart Disease
InactiveUS20200360391A1Improve complianceOrganic chemistryEster active ingredientsPhosphodiesteraseEnzyme Inhibitor Agent
Owner:OSAKA UNIV +1
Compositions and methods involving the combination of a thromboxane A2 receptor antagonist and an inhibitor of cyclooxygenase-1
InactiveUS20050059741A1Improve efficiencyReduce riskBiocideElcosanoid active ingredientsThromboxane A2 receptorDepressant
The invention is directed to methods and compositions that can be used in the treatment of inflammation, pain, and cardiovascular disorders. Methods and compositions are described involving the combination of a thromboxane A2 receptor antagonist and an inhibitor of cyclooxygenase-1.
Owner:B M R A CORP
Method of using niclosamide derivatives
ActiveUS11260034B2Prevent agglutinationPreventing cardiovascular diseasesOrganic active ingredientsHydrolysed protein ingredientsDiseaseThrombus
Niclosamide derivatives are provided in the present invention. More particularly, the methods of using niclosamide derivatives for the manufacture of medicaments for suppressing platelet aggregation and preventing thrombosis-related diseases are provided. The niclosamide derivatives in the medicaments inhibit the production of thromboxane A2, therefore suppress platelet aggregation and prevent thrombosis-related diseases.
Owner:CHANG GUNG UNIVERSITY
Application of Trilenin Component C in Sanleng in the Preparation of Medicines for Anti-blood Stasis Syndrome
ActiveCN109172577BSignificant anti-blood stasis effectImprove liver indexOrganic active ingredientsBlood disorderDepressantEndothelin 1
The invention discloses application of a scirpusin component C in common burreed rhizome in synthesis of anti-blood stasis syndrome drugs. The invention first proves that the scirpusin C has an obvious anti-blood stasis drug effect, and can be used for preparing a medicine for treating the blood stasis syndrome. Pharmacological experiments prove that the scirpusin C is capable of obviously reducing the levels of thromboxane B2 (TXB2), fibrinogen (FIB), active plasminogen activator inhibitor (PAI-1) and endothelin 1 (ET-1) in serum of blood stasis model mice and improving the thymus index (TI),spleen index (SI) and hepatic index (HI) of the mice, and has high clinical application value and development prospects in the aspect of treating the blood stasis syndrome.
Owner:GUANGDONG PHARMA UNIV
Pharmaceutical composition comprising a 4-hydroxy-2-oxo-2, 3- dihydro-1, 3-benzothiazol-7-yl compound for modulation of beta2-adrenoreceptor activity
InactiveCN102131505AOrganic active ingredientsRespiratory disorderAntioxidantLipoxygenase Inhibitors
The invention provides a pharmaceutical product, kit or composition comprising a first active ingredient which is N-Cyclohexyl-N3-(2-(3-fluorophenyl)ethyl)-N-(2-[(2-(4- hydroxy-2-oxo-2,3-dihydro- 1,3-benzothiazol-7-yl)ethyl)amino] ethyl)-beta-alaninamide or a salt thereof, and a second active ingredient selected from: a non-steroidal Glucocorticoid Receptor (GR Receptor) Agonist; an antioxidant; a CCRl antagonist; a chemokine antagonist (not CCRl); a corticosteroid; a CRTh2 antagonist; a DPI antagonist; an Histone Deacetylase Inducer; an IKK2 inhibitor; a COX inhibitor; a lipoxygenase inhibitor; a leukotriene receptor antagonist; an MPO inhibitor; a muscarinic antagonist; a p38 inhibitor; a PDE inhibitor; a PPAR<gamma> agonist; a protease inhibitor; a Statin; a thromboxane antagonist; a vasodilator; or, an ENAC blocker (Epithelial Sodium-channel blocker); and its use in the treatment of respiratory disease (for example chronic obstructive pulmonary disease (COPD) or asthma); to certain salts of N-Cyclohexyl-N3-(2-(3-fluorophenyl)ethyl)- N-(2-[(2-(4-hydroxy-2-oxo-2,3-dihydro-l,3-benzothiazol-7-yl)ethyl)amino]ethyl)-beta- alaninamide and to an intermediate useful in the manufacture of this pharmaceutically active substance and salts thereof.
Owner:ASTRAZENECA AB
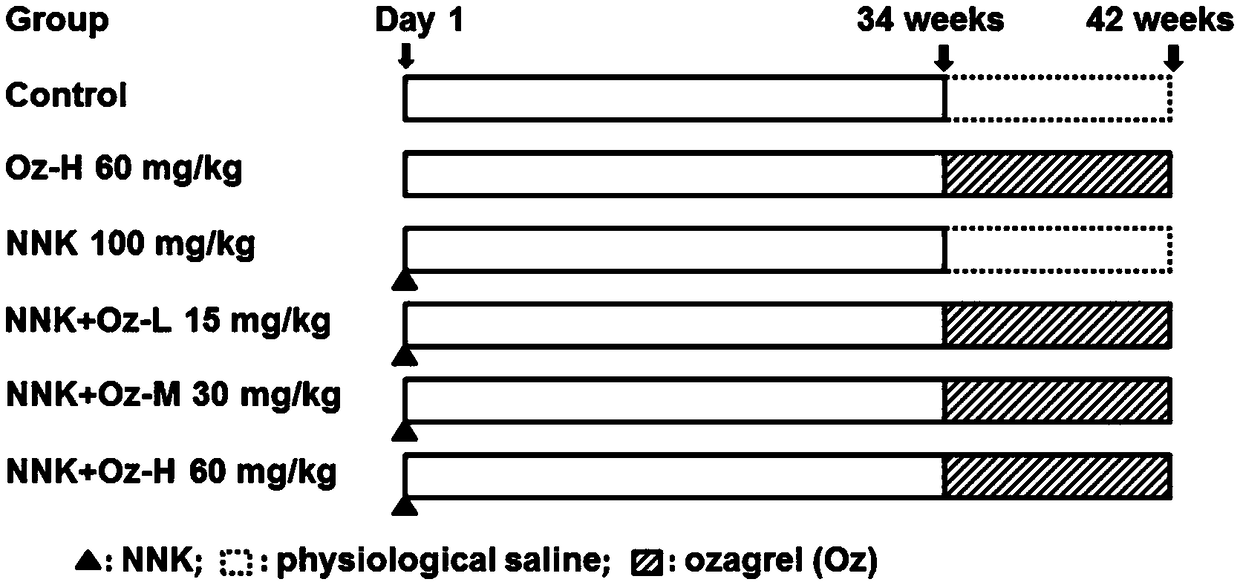
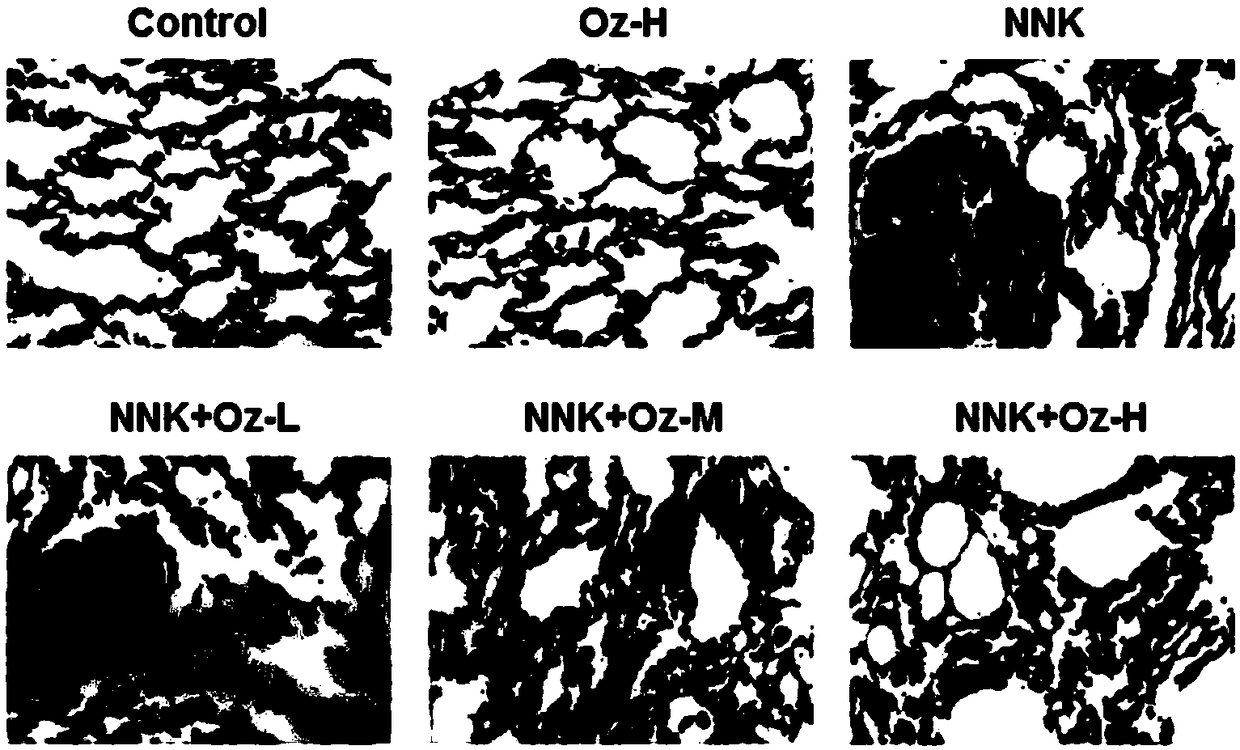
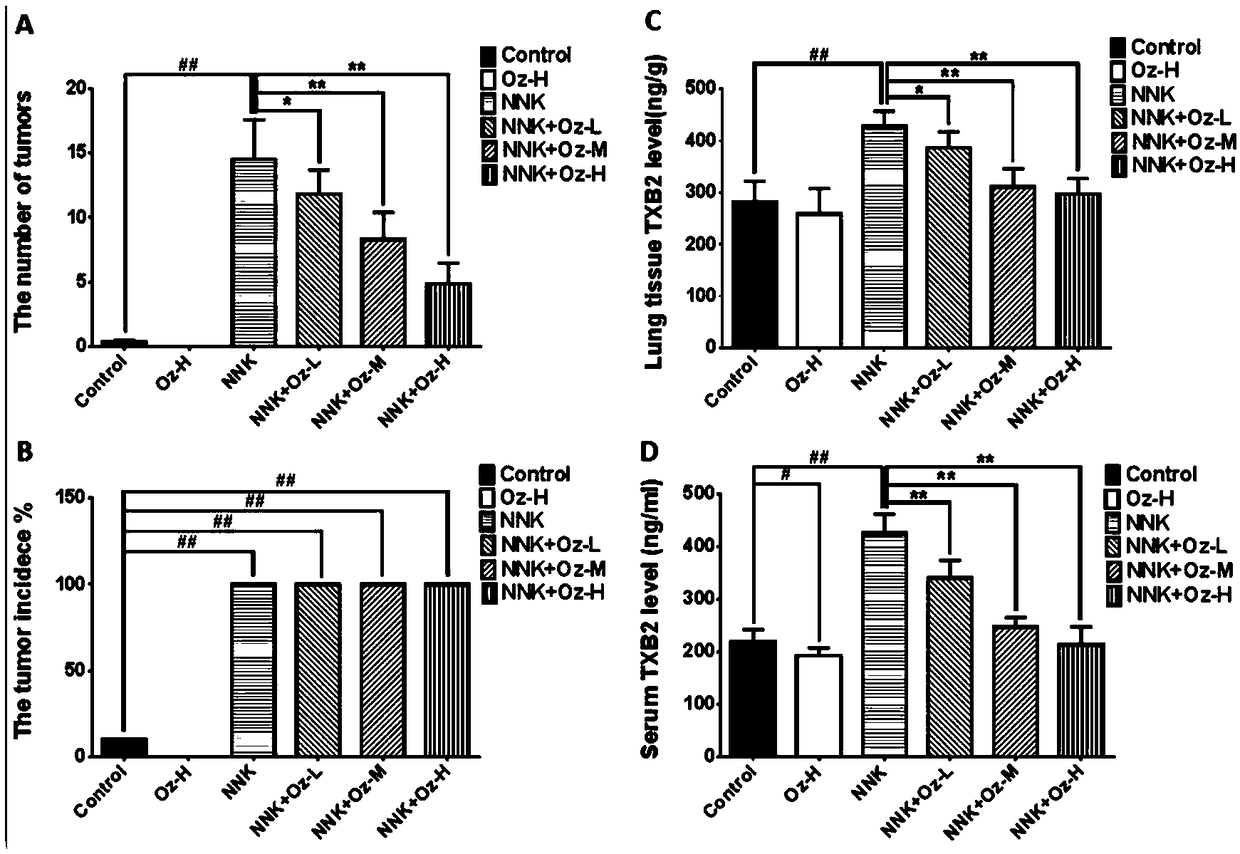
Application of thromboxane A2 synthetase inhibitor ozagrel in preparing drug for treating smoking-induced lung cancer
InactiveCN109481436AReduce sizeTumor size reductionOrganic active ingredientsAntineoplastic agentsLung cancerEnzyme inhibitor
The invention provides an application of thromboxane A2 synthetase inhibitor ozagrel in preparing a drug for treating smoking-induced lung cancer. It is found that the thromboxane A2 synthetase has apotential to become a novel target for treating lung cancer. Ozagrel reduce the level of thromboxane A2 by inhibiting the activity of the thromboxane A2 synthetase so as to reduce the size of lung cancer induced by smoking obviously, thereby providing a novel application path for ozagrel.
Owner:GUANGDONG MEDICAL UNIV
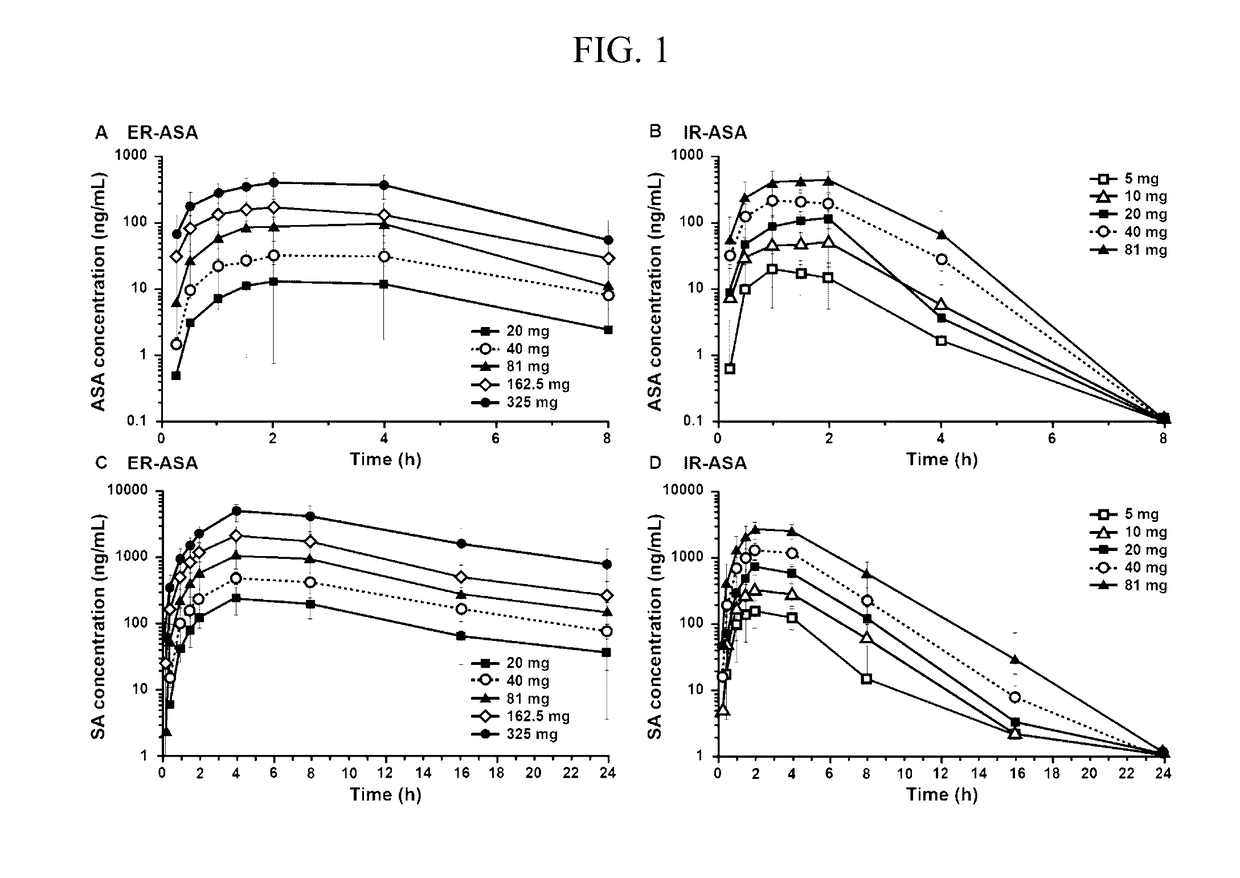
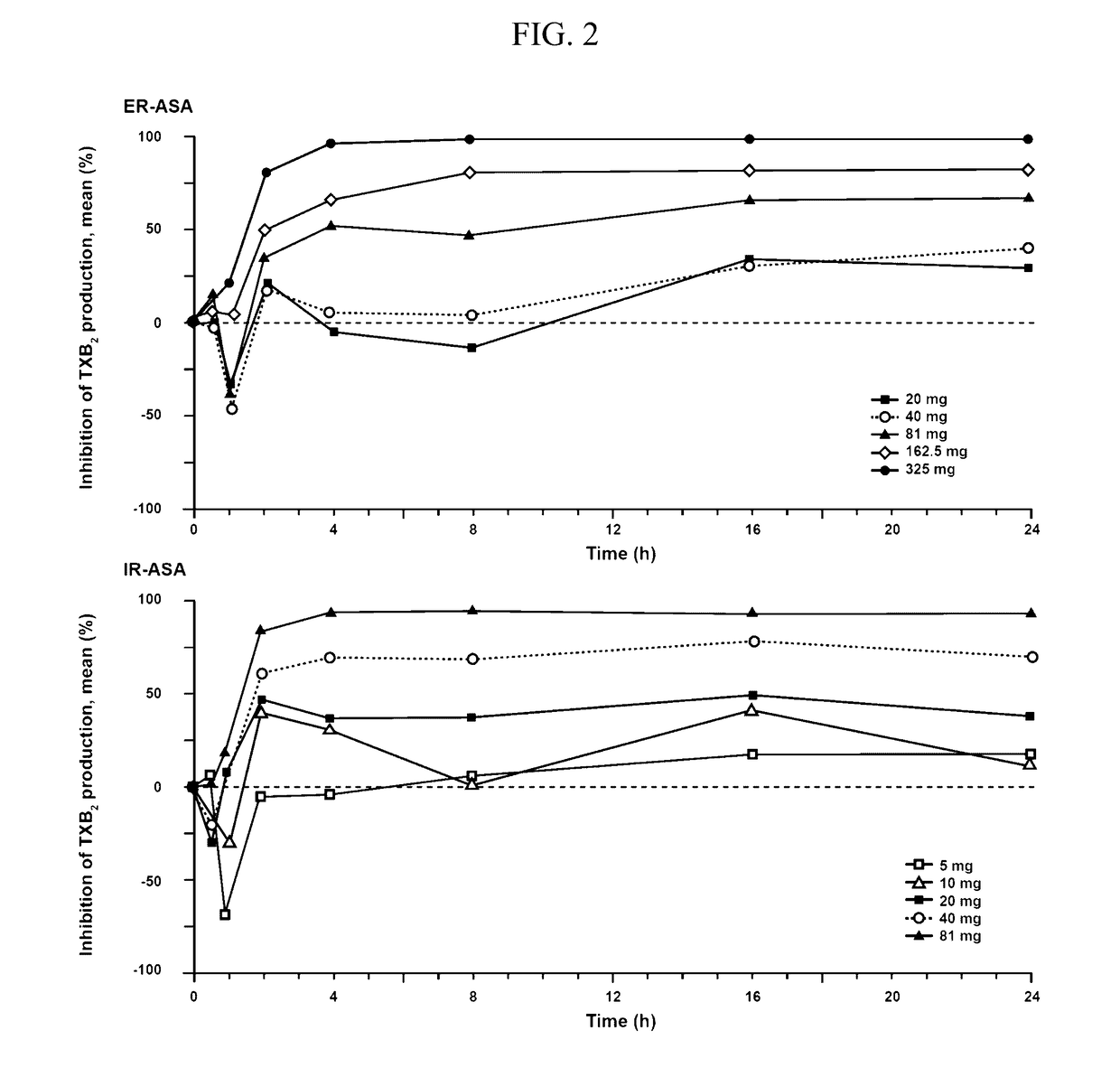
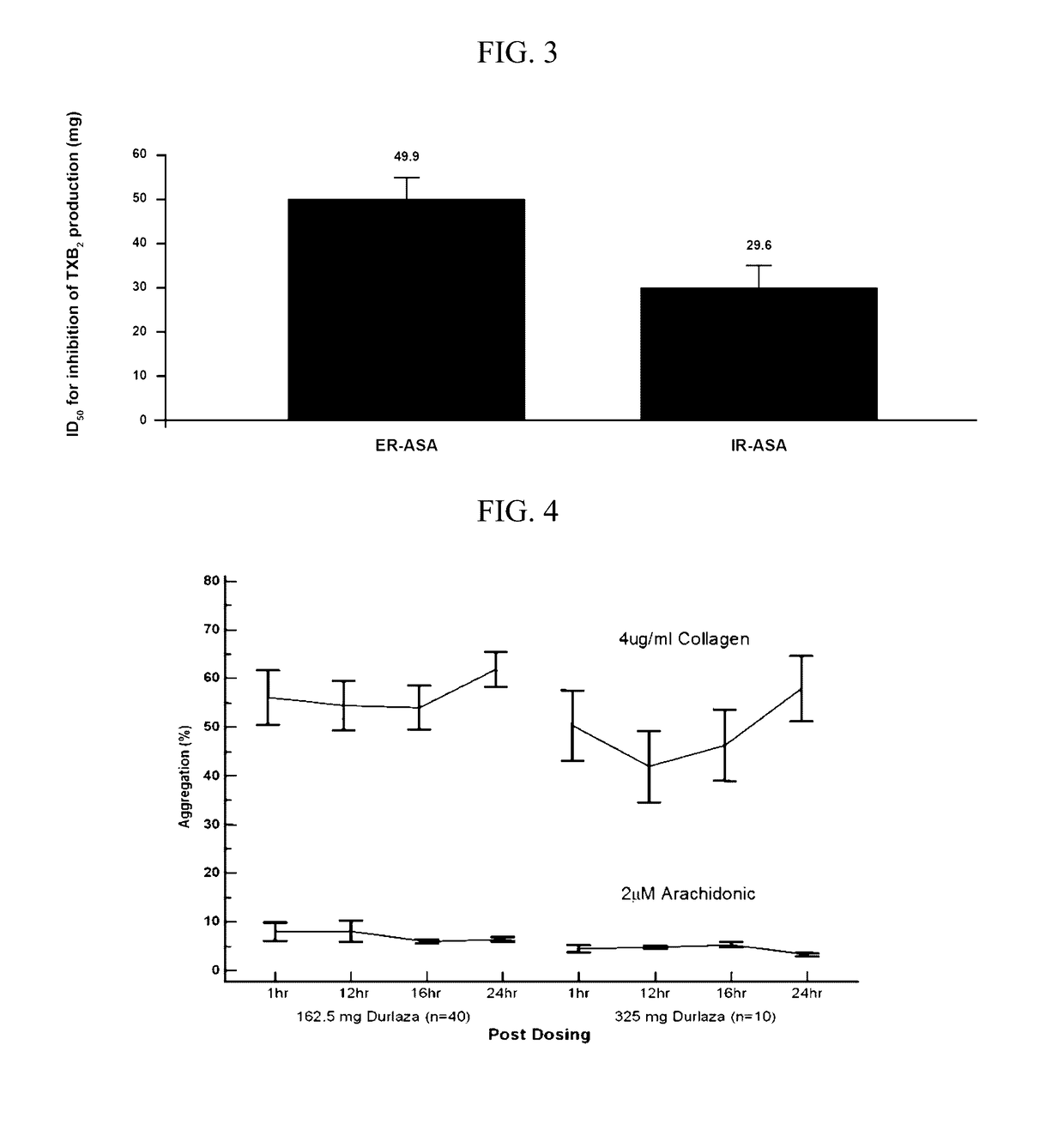
Extended Release Aspirin
InactiveUS20170112857A1Reducing serum thromboxane B levelReduced serum thromboxane B levelOrganic active ingredientsPharmaceutical delivery mechanismCancer preventionImmediate release
The present invention is directed to methods of inhibiting platelet aggregation, reducing serum thromboxane B2 levels, reducing systemic or cardiovascular inflammation, treating or preventing cancer and treating or preventing cardiovascular disease by oral administration of compositions containing extended release acetylsalicylic acid (ASA) or a combination of extended release ASA and immediate release ASA.
Owner:CADRENAL THERAPEUTICS
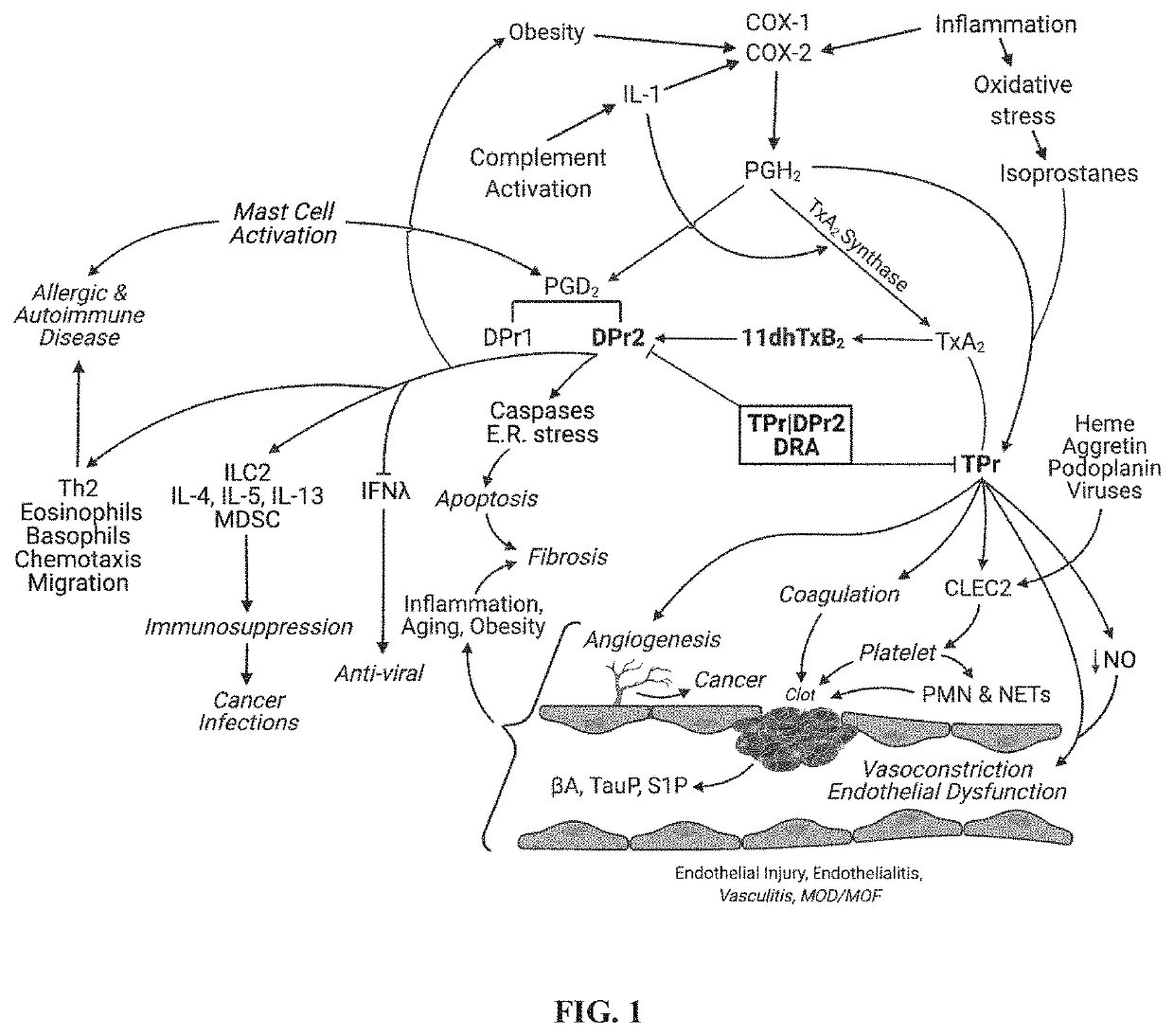
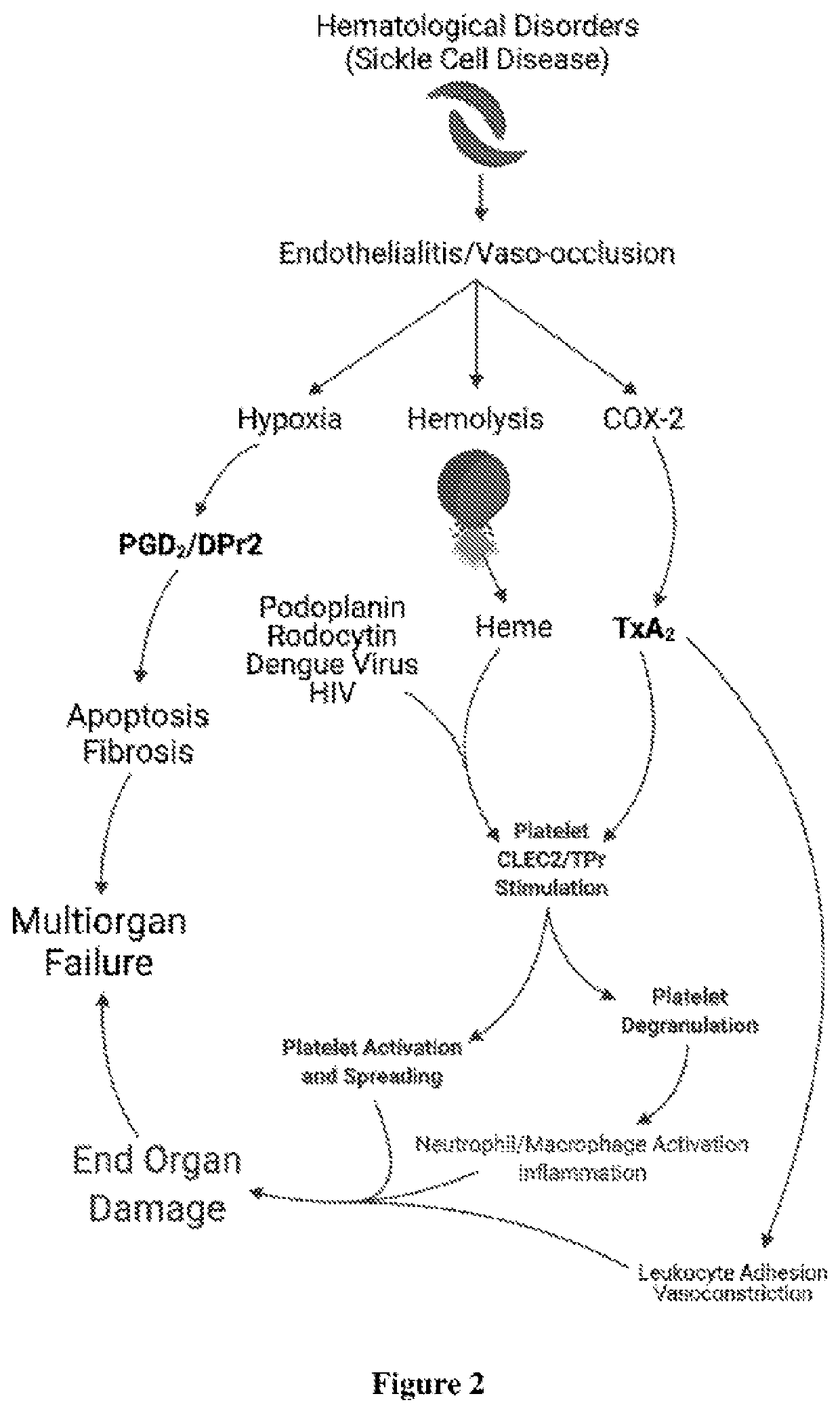
Dual antagonist of pgd2/dpr2 and thromboxane a2/tpr receptors and use for treatment of maladaptive immune response or thrombotic diathesis
ActiveUS20220184031A1Reduce inflammationReduce penetrationRespiratory disorderAntineoplastic agentsDiseaseProstaglandins D
The present invention relates to a pharmaceutical composition for treatment of a disease or condition characterized by or associated with stimulation of both DPr2 and TPr signaling, said composition comprising an effective amount of a dual receptor antagonist of DPr2 for prostaglandin D2 and TPr for thromboxane A2 and a pharmaceutically acceptable carrier.
Owner:APPL MEDICAL TECH LLC
Application of trilensin component a in sanleng in the preparation of anti-blood stasis medicine
ActiveCN108926566BSignificant anti-blood stasis effectImprove liver indexOrganic active ingredientsBlood disorderSpleenPharmacology
The invention discloses an anti-blood stasis syndrome application of a scirpusin A component in Rhizoma Sparganii. It is firstly proved that the scirpusin A has a significant anti-blood stasis drug effect, and can be used to prepare drugs for treating blood stasis syndromes. Pharmacological experiments prove that the scirpusin A can significantly reduce the Thromboxane B2 (TXB2) level, the fibrinogen (FIB) level, the tissue plasminogen inhibitor-1 (Active Plasminogen Activator Inhibitor-1, PAI-1) level and the endothelin (ETo-1) level in serum of a blood stasis model mouse, can improve the thymus index (TI), the spleen index (SI) and the hepatic index (HI) index of the mouse, and has high clinical application values and a broad development prospect in the treatment of blood stasis syndromes.
Owner:GUANGDONG PHARMA UNIV
Detection kit for detecting aspirin metabolism ability and preparation method thereof
ActiveCN114002433BImprove capture efficiencyHigh detection sensitivityMaterial analysis by observing effect on chemical indicatorDisease diagnosisAnti plateletImmuno detection
The invention discloses a detection kit for detecting the metabolic capacity of aspirin and a preparation method thereof, wherein the kit includes a 11-dehydrothromboxane B2 monoclonal antibody-coated microtiter plate, a 11-dehydrothromboxane B2 Thromboxane B2 competitor, chromogenic substrate, stop solution and 11-dehydrothromboxane B2 standard solution, the monoclonal antibody of 11-dehydrothromboxane B2 is mouse monoclonal antibody, and the light chain variable region of mouse monoclonal antibody The sequence is shown in SEQ ID NO.2 of the sequence listing, and the sequence of the heavy chain variable region of the mouse monoclonal antibody is shown in SEQ ID NO.4 of the sequence listing. The invention adopts an enzyme-linked immunosorbent detection kit and its method to detect aspirin metabolism so as to judge whether a patient has aspirin resistance or resistance. It has the characteristics of accuracy and high sensitivity, and is not easily affected by biochemical indicators such as creatinine. Failure or ineffectiveness of antiplatelet aggregation therapy due to aspirin resistance.
Owner:湖南菲思特精准医疗科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Pharmaceutical composition comprising a dual antagonist against PGD<sub>2</sub>/TXA<sub>2 </sub>receptors having a [2.2.1] or [3.1.1] bicyclic skeleton Pharmaceutical composition comprising a dual antagonist against PGD<sub>2</sub>/TXA<sub>2 </sub>receptors having a [2.2.1] or [3.1.1] bicyclic skeleton](https://images-eureka.patsnap.com/patent_img/de12c428-7830-4569-ae75-94e511a1e45d/US07105564-20060912-C00001.png)
![Pharmaceutical composition comprising a dual antagonist against PGD<sub>2</sub>/TXA<sub>2 </sub>receptors having a [2.2.1] or [3.1.1] bicyclic skeleton Pharmaceutical composition comprising a dual antagonist against PGD<sub>2</sub>/TXA<sub>2 </sub>receptors having a [2.2.1] or [3.1.1] bicyclic skeleton](https://images-eureka.patsnap.com/patent_img/de12c428-7830-4569-ae75-94e511a1e45d/US07105564-20060912-C00002.png)
![Pharmaceutical composition comprising a dual antagonist against PGD<sub>2</sub>/TXA<sub>2 </sub>receptors having a [2.2.1] or [3.1.1] bicyclic skeleton Pharmaceutical composition comprising a dual antagonist against PGD<sub>2</sub>/TXA<sub>2 </sub>receptors having a [2.2.1] or [3.1.1] bicyclic skeleton](https://images-eureka.patsnap.com/patent_img/de12c428-7830-4569-ae75-94e511a1e45d/US07105564-20060912-C00003.png)