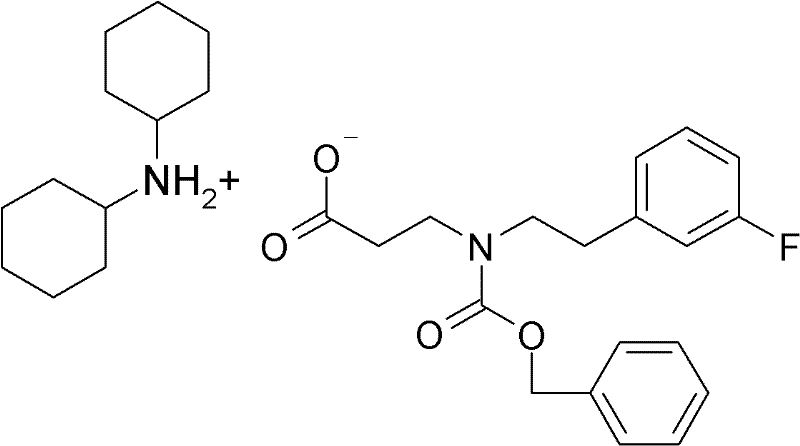
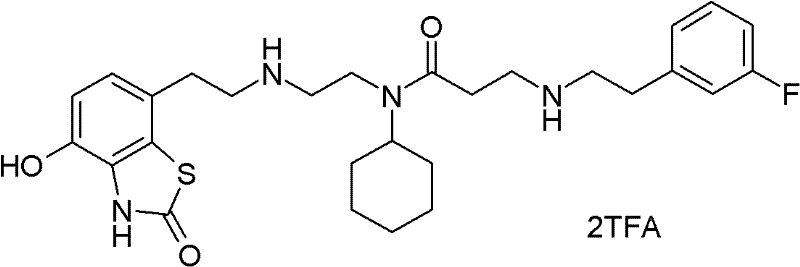
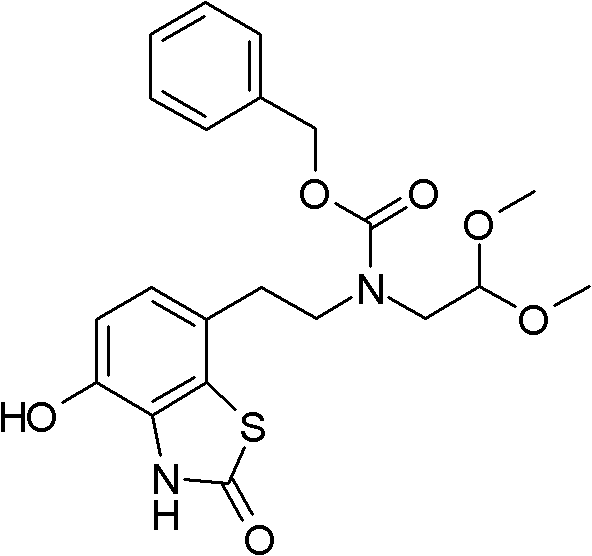
Pharmaceutical composition comprising a 4-hydroxy-2-oxo-2, 3- dihydro-1, 3-benzothiazol-7-yl compound for modulation of beta2-adrenoreceptor activity
A technology of cycloheptane carbonyloxy and hydroxyl, which is applied in the field of intermediates for the preparation of the pharmaceutical active substance and its salt, and can solve problems such as unsatisfactory efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0509] Samples of material obtained by preparation 16 as described above in this application were analyzed by XRPD (PANalytical X'Pert or Cubix system), GVS and DSC. Melting point by DSC was 189°C (onset) (±2°C), GVS determined at 0.1% weight gain (%w / w) at 80% relative humidity (±0.2%).
[0510] (R)-1-(4-fluorophenethyl)-3-((S)-2-phenyl-2-(piperidin-1-yl)propionyloxy)-1- prepared according to Preparation 16 The XRPD spectrum of azonia bicyclo[2.2.2]octane 4-methylbenzenesulfonate is shown in FIG. 28 .
[0511] Figure 28
[0512]
[0513] cAMP production mediated by adrenergic β2
[0514] cell preparation
[0515] at 225cm 2 Flask incubator (37°C and 5% CO 2 ) in RPMI medium (containing 10% (v / v) FBS (fetal bovine serum) and 2 mM L-glutamine).
[0516] experimental method
[0517] Attached H292 cells were removed from tissue culture flasks as follows: with Accutase TM Cell detachment solution was treated for 15 minutes. Place the flask in a humidified incub...
Embodiment 1
[0543] Evaluation of compound activity on intraalveolar neutrophil migration following aerosol challenge with lipopolysaccharide (LPS) in CRL:CD rats
[0544] LPS challenge in CRL:CD rats brought inflammatory cells into the lungs. Rats were challenged with 0.9% w / v saline aerosol for 30 minutes or with 0.1 mg / ml [LPS] / [0.9% saline] aerosol challenge for 30 minutes or intratracheal administration of 0.1-10 μg / kg. This was repeated up to 8 times according to the experimental protocol. Based on the experimental protocol, rats are dosed with vehicle, standard compound or test compound by appropriate route and frequency at different time points pre-challenge and post-challenge. Panels of test compounds can be different doses of the same compound or single doses of different compounds or a combination of both. Test compounds are administered by intraperitoneal, intravenous or subcutaneous injection or by inhalation or intratracheal administration.
[0545] Depending on the nature...
Embodiment 2
[0548] Evaluation of compound activity on intraalveolar neutrophil migration following aerosol challenge with lipopolysaccharide (LPS) in guinea pigs
[0549] Male Dunkin-Hartley guinea pigs (300-600 g) were placed in open-fronted guinea pig holding cones connected randomly around a cylindrical aerosol chamber. Each group of guinea pigs was maintained in a challenge cone and exposed to either a vehicle aerosol or a 0.1-30 [mu]g / ml [LPS] / [0.9% saline] aerosol. Aerosols were generated using 2 jet nebulizers per column with a flow rate of 12 L / min. 10ml of elicitor was placed in each nebulizer. Alternatively, 0.1-10 [mu]g / kg is administered intratracheally to the animal. This was repeated up to 8 times according to the experimental protocol.
[0550] Based on the experimental protocol, guinea pigs are dosed with vehicle, standard compound or test compound at the appropriate route and frequency at different time points before and after challenge. Panels of test compounds can b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com