Method for researching pharmacodynamic relationship of various medicinal components of compound thromboxane preparation
A technology of compound Xueshuantong and medicinal materials, which is applied in the field of research on the relationship between the medicinal effects of various medicinal materials in compound Chinese medicine prescriptions, can solve the problems of lack of connotation of the prescription, contribution of medicinal effects, primary and secondary, and unclear interaction relations, etc., and achieve good practical value Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
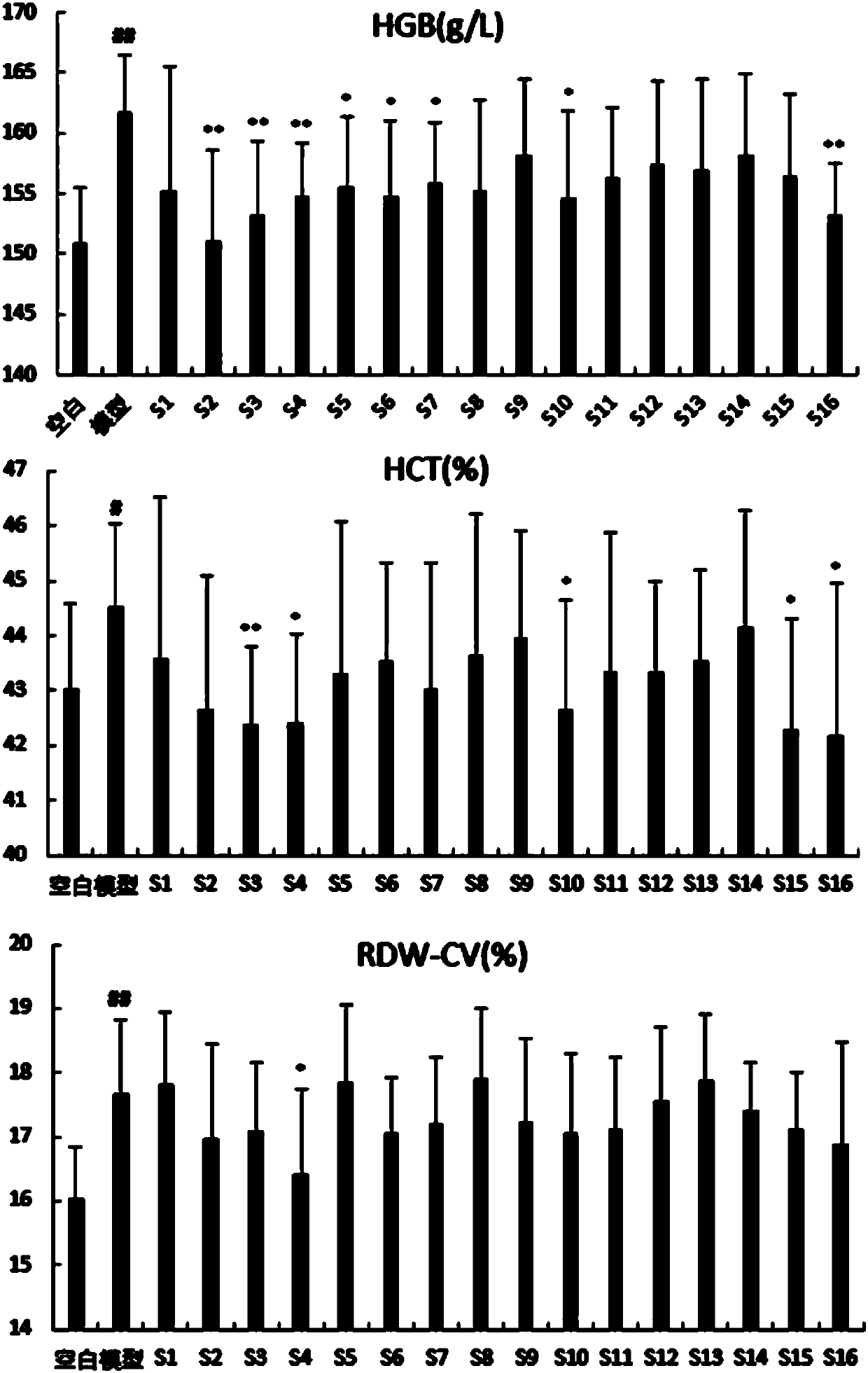
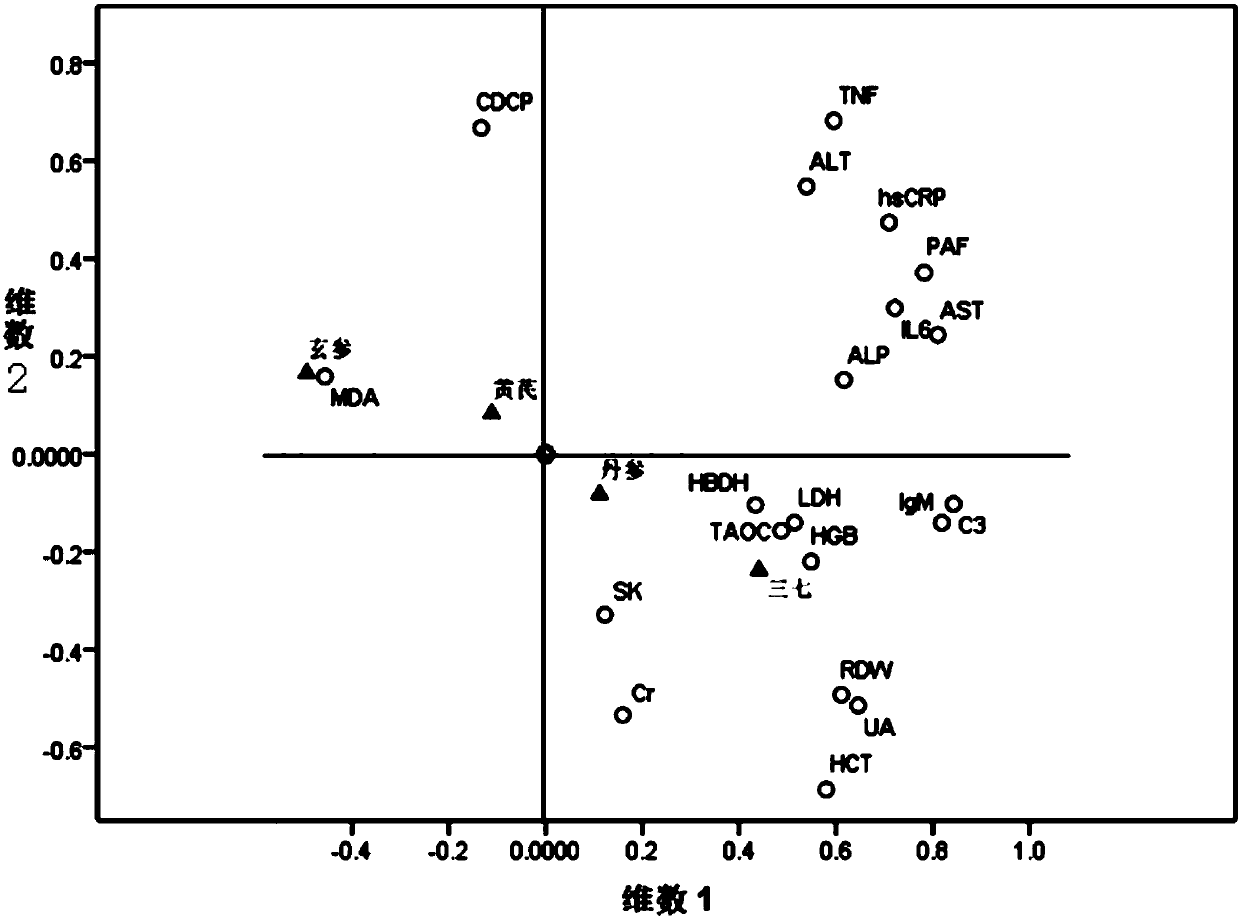
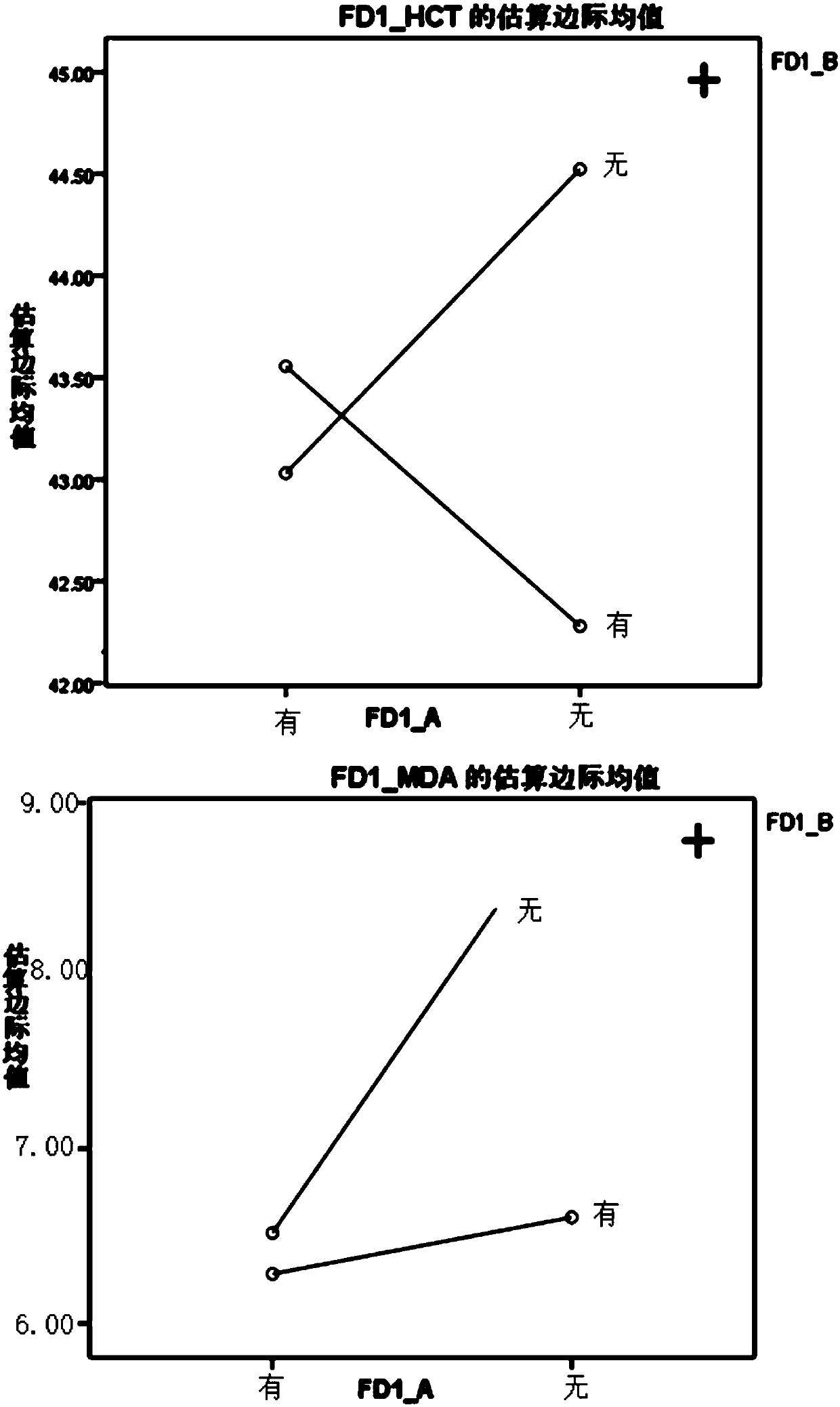
[0030] Example: The contribution, primary and secondary, and interaction relationship of Sanqi, Danshen, Astragalus, and Scrophulariaceae in compound Xueshuantong preparations
[0031] 1. Materials
[0032] (1) Animals: 180 SPF (specific-pathogen free) male SD (Sprague Dawley) rats, weighing 180-220g, animal quality certificate number: 44007200034125; the animals were provided by the Guangdong Provincial Medical Experimental Animal Center, and the animal facility license Certificate number: SCXK-(Guangdong) 2013-0002.
[0033] (2) Instruments: Roche P 800 automatic biochemical analyzer (Roche, MODULAR P800), Mindray BC-5800 automatic blood cell analyzer (Mindray, BC-5800), low temperature centrifuge (Eppendorf, 5430R), 100,000 points One electronic balance (Sartorius, BP211D), ultra-low temperature refrigerator (Haier, BCD-568W), HH-4 digital display constant temperature water bath (Guohua Electric Co., Ltd., HH-4), DG5033A microplate reader (Nanjing Huadong Electronics Group...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com