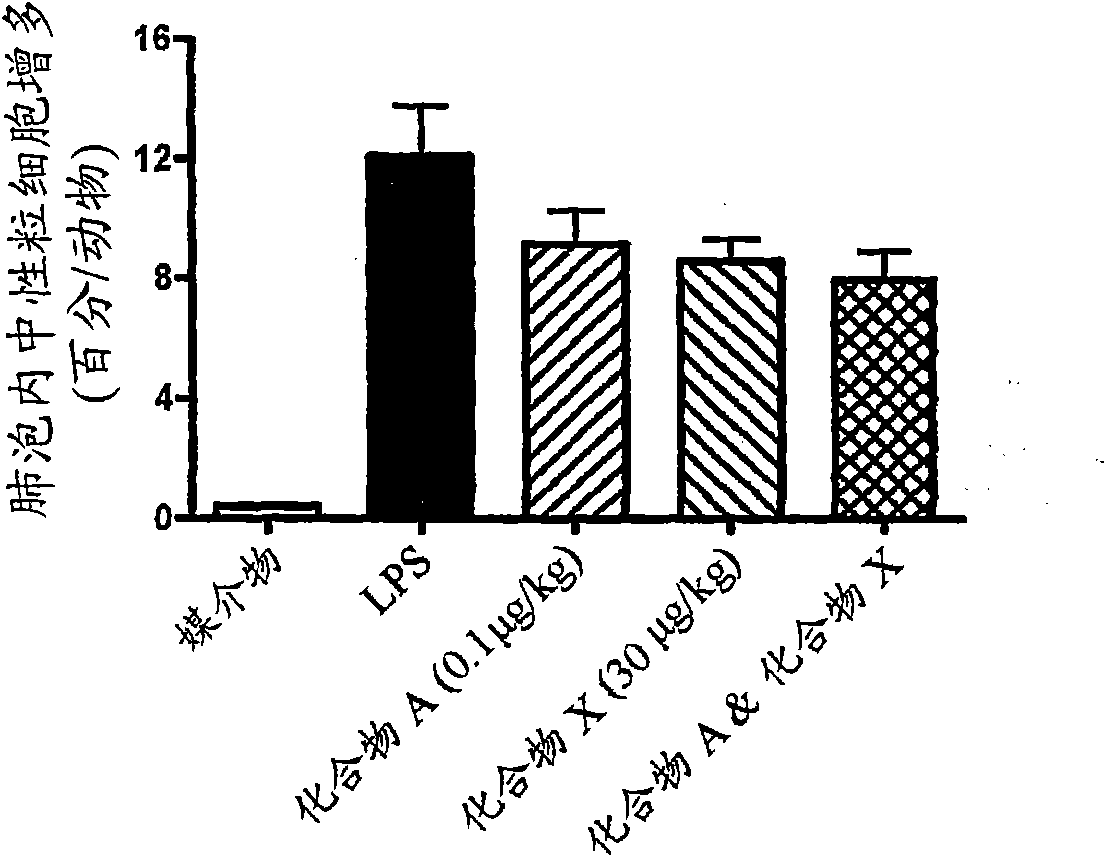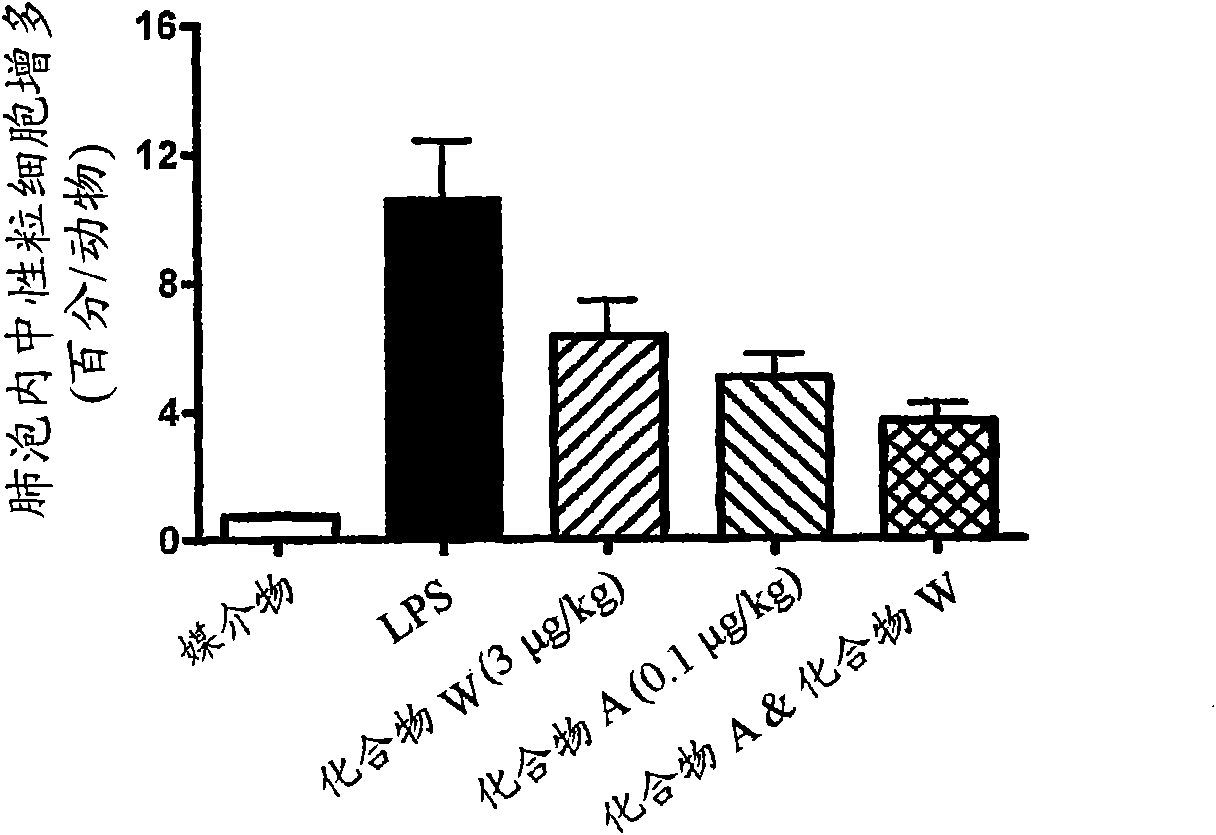Combinations of beta- 2 -adrenoceptor agonistic benzothiazolone
A receptor antagonist and receptor technology, applied in the field of combination of beta-2 adrenergic receptor antagonistic benzothiazolones, can solve the problems of unsatisfactory efficacy and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
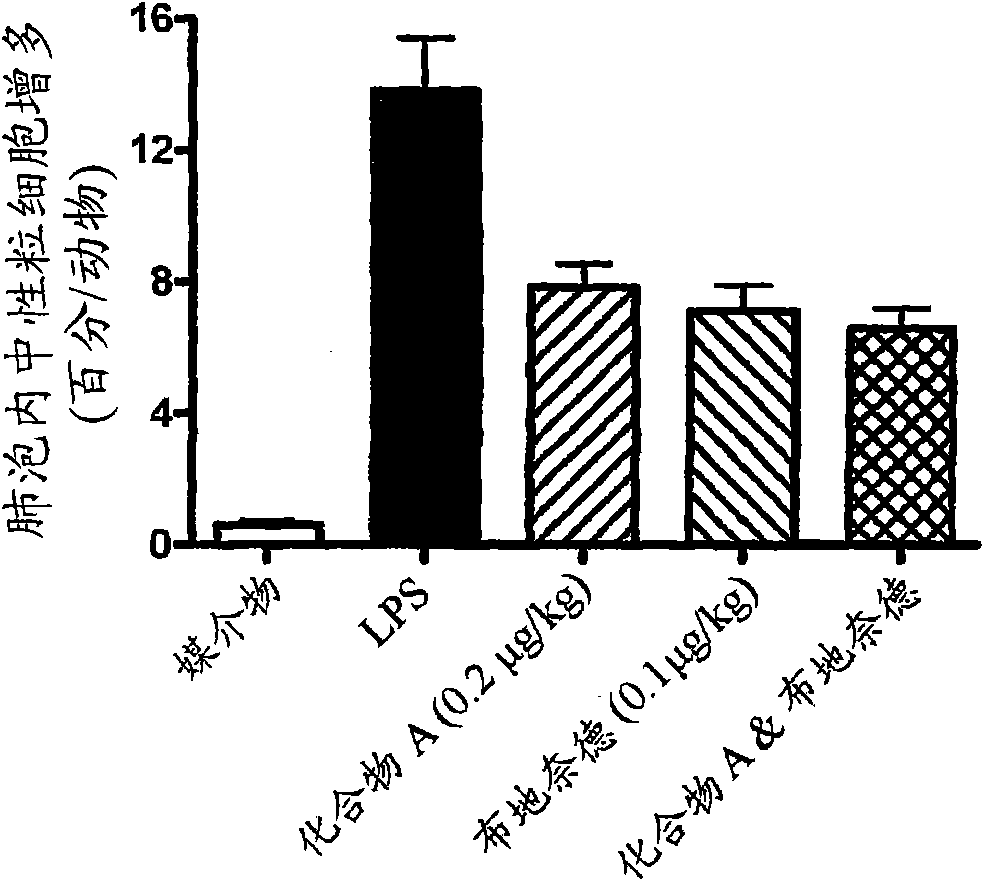
[0241] For the effect of compounds on lung after intratracheal challenge with lipopolysaccharide (LPS) in CRL:CD rats Evaluation of activity of neutrophil migration in vesicles
[0242] LPS challenge in CRL:CD rats induces neutrophil influx into the lungs. Under recoverable gas (5% isoflurane / oxygen) anesthesia, rats were administered vehicle (0.05M phosphate, 0.1% Tween 80, 0.6% saline, pH 6) or compound via the intratracheal route , challenged 30 minutes later with LPS at an intratracheal dose of 10 μg / kg.
[0243] Rats (250-400 g) were euthanized with 1 mL sodium pentobarbital 4 hours after LPS challenge. A tracheostomy was performed and intubated. The airways were then lavaged with 3 mL of Isoton at room temperature. Isoton (Beckman Coulter, High Wycombe, UK) was left in the airway for 10 seconds and then removed. Bronchiolo-alveolar lavage (BAL) fluid containing inflammatory cells was placed in a 15 mL centrifuge tube and kept on ice. This operation was repeated ...
Embodiment 2
[0251] For compounding after aerosol challenge with lipopolysaccharide (LPS) in guinea pigs Evaluation of the Activity of Drugs on the Migration of Intraalveolar Neutrophils
[0252] Male Dunkin-Hartley guinea pigs (300-600 g) were placed in an open-fronted guinea pig holding cone attached randomly around the perimeter of a cylindrical aerosol chamber. Each group of guinea pigs was maintained in a challenge cone and exposed to vehicle spray or LPS at a concentration of 0.1-30 [mu]g / ml in 0.9% saline. Two sprayers per column were used to generate the spray with a flow rate of 12 L / m. 10ml of challenger was placed in each nebulizer. Alternatively animals receive an intratracheal dose of 0.1-10 [mu]g / kg. This was repeated up to 8 times according to the experimental protocol.
[0253] Depending on the experimental protocol, guinea pigs are dosed with vehicle, standard compound or test compound at the appropriate route and frequency at various time points before and after ch...
Embodiment 3
[0255] Effects of compounds on alveolar neutrophils after aerosol challenge with lipopolysaccharide (LPS) in mice Evaluation of Cell Migration Activity
[0256] Male C57BL / 6 / J or BALB / C mice (20-35 g) were housed in groups of up to 20 in Perspex exposure boxes and exposed to 0.3 mg / ml LPS spray or 0.9% w / v In saline spray. LPS (Sigma, E. coli, reference L-3755, serotype 026:B6, lot 111k4078) was made in 0.9% w / v saline. Nebulization was generated using two nebulizers operating at a flow rate of 12 L / min (6 L / min for each nebulizer) for 15 minutes. Alternatively animals receive an intratracheal dose of 0.1-10 [mu]g / kg. This was repeated up to 8 times according to the experimental protocol.
[0257] Depending on the experimental protocol, mice are dosed with vehicle, standard compound or test compound by appropriate route and frequency at various time points before and after challenge. The test compound panel can be different doses of the same compound or a single dose o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com