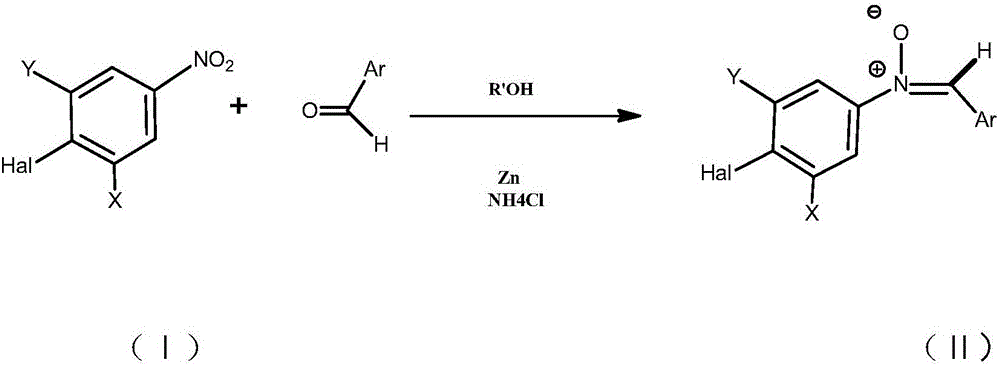
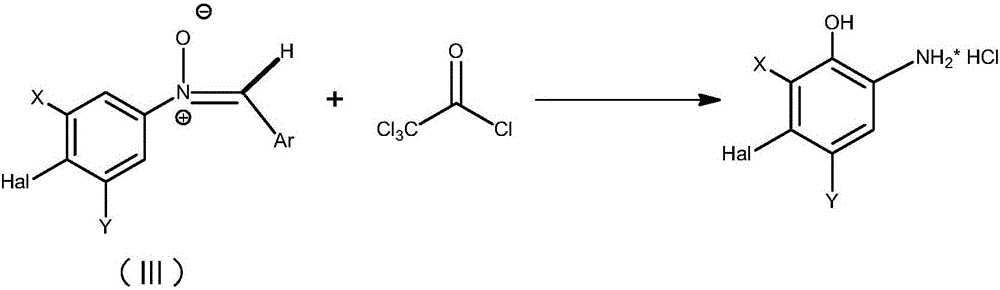
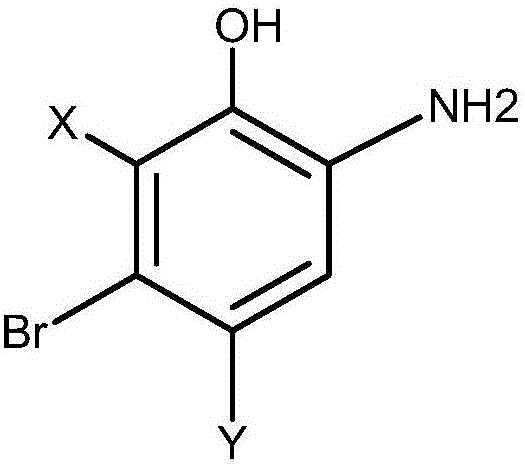
Preparation method of 2-amino-5-halogen phenol compounds
A technology for halogenated phenols and compounds is applied in the field of preparation of pharmaceutical intermediates, and can solve the problems of lack of large supply, high price and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0018] Through the following specific embodiments, the present invention is further explained and described in detail, so that those of ordinary skill in the art can understand the present invention more easily, but it should not be interpreted as that the scope of the subject matter described in the present invention is limited to the following examples and limitations on any or all rights in the invention. Without substantive changes in technical content, it should also be regarded as the scope of the present invention that can be implemented.
[0019] [A]; 2-Amino-5-bromophenol hydrochloride
[0020] Step (1): Preparation of N-(4-bromo)-α-phenylnitrone
[0021] Under nitrogen protection, in a 100mL three-necked round-bottom flask equipped with a magnetic stirring rotor, a thermometer, and a constant pressure dropper, sequentially add 4-bromonitrobenzene (5.05g, 0.025mol), methanol (25mL) and 15ml Benzaldehyde (2.92 g, 0.0275 mol) dissolved in methanol was stirred at room ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com