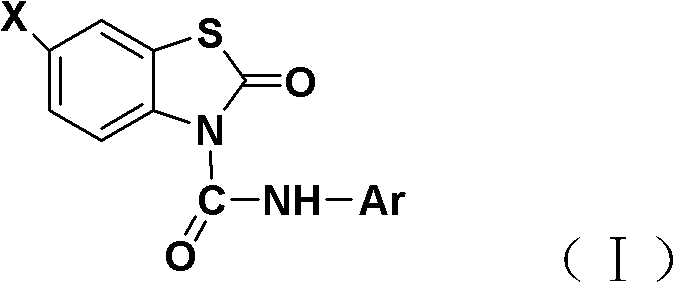
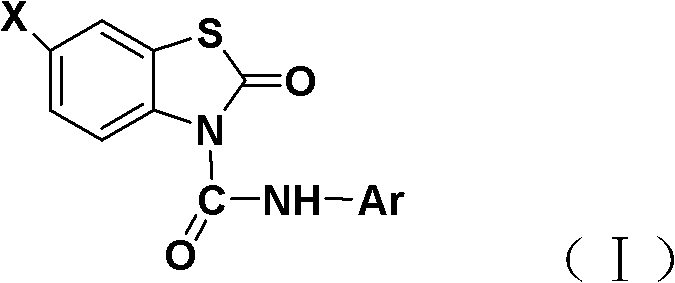
N-substituted phenyl-benzothiazolone-3-formamide derivative
A technology of benzothiazolone and formamide, applied in biocides, chemicals for biological control, biocides, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The synthesis of embodiment 1N-phenyl-benzothiazolone-3-carboxamide
[0024] Benzothiazolone (1.52 g, 10 mmol) and triethylamine (1 mmol) were dissolved in 20 mL of dichloromethane and stirred. Under cooling in an ice bath, phenylisocyanate (12 mmol) was added dropwise. After the dropwise addition was completed, the reaction was carried out at 0-3°C. The reaction was tracked and monitored by TLC. After the reaction was completed for 10 hours, the reaction solution was concentrated, and the residue was recrystallized with 10 mL of absolute ethanol to obtain white crystals, that is, N-phenyl-benzene And thiazolone-3-carboxamide. The melting point is 162-164°C, and the yield is 86.0%.
[0025] of the compound 1 H NMR and IR are as follows,
[0026] 1 H NMR (DMSO-d 6 , 500MHz) δ: 7.11~7.59 (m, 9H, -Ph), 8.92 (s, 1H, -NH); IR (KBr) v: 3311, 1719, 1670, 1551, 1503, 1439, 1267cm -1 .
Embodiment 2
[0027] The synthesis of embodiment 2N-phenyl-6-chloro-benzothiazolone-3-carboxamide
[0028] 6-Chloro-benzothiazolone (1.87 g, 10 mmol) and 4-dimethylaminopyridine (1 mmol) were dissolved in 30 mL of toluene and stirred. Under cooling in an ice bath, phenylisocyanate (12 mmol) was added dropwise. After the dropwise addition was completed, the reaction was carried out at 0-3°C, and the reaction was followed and monitored by TLC. After the reaction was completed for 12 hours, the reaction liquid was concentrated, and the residue was recrystallized with 15 mL of n-hexane to obtain white crystals, namely N-phenyl-6-chloro -Benzothiazolone-3-carboxamide. The melting point is 182-184°C, and the yield is 84.0%.
[0029] of the compound 1 H NMR and IR are as follows,
[0030] 1 H NMR (DMSO-d 6 , 500MHz) δ: 7.02~7.99 (m, 8H, -Ph), 10.08 (s, 1H, -NH); IR (KBr) v: 3268, 1731, 1642, 1479, 1391, 1300, 1259cm -1 .
Embodiment 3
[0031] The synthesis of embodiment 3N-p-tolyl-benzothiazolone-3-carboxamide
[0032] Benzothiazolone (1.52 g, 10 mmol) and pyridine (1 mmol) were dissolved in 30 mL of dichloromethane and stirred. Under cooling in an ice bath, p-tolyl isocyanate (12 mmol) was added dropwise. After the dropwise addition was completed, the reaction was carried out at 3 to 5°C, and the reaction was followed and monitored by TLC. After the reaction was completed for 10 hours, the reaction solution was concentrated, and the residue was recrystallized with 10 mL of ethyl acetate to obtain white crystals, namely, N-p-tolyl- Benzothiazolone-3-carboxamide. The melting point is 173-175°C, and the yield is 82.0%.
[0033] of the compound 1H NMR and IR are as follows,
[0034] 1 H NMR (DMSO-d 6 , 500MHz) δ: 2.29 (s, 3H, -CH3), 7.09~8.38 (m, 8H, -Ph), 8.88 (s, 1H, -NH);
[0035] IR (KBr) v: 3298, 1729, 1668, 1569, 1547, 1439, 1288cm -1 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com