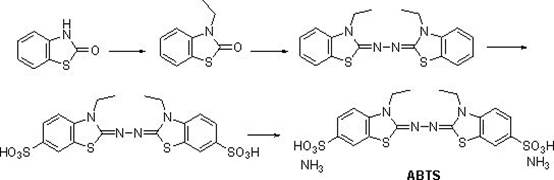
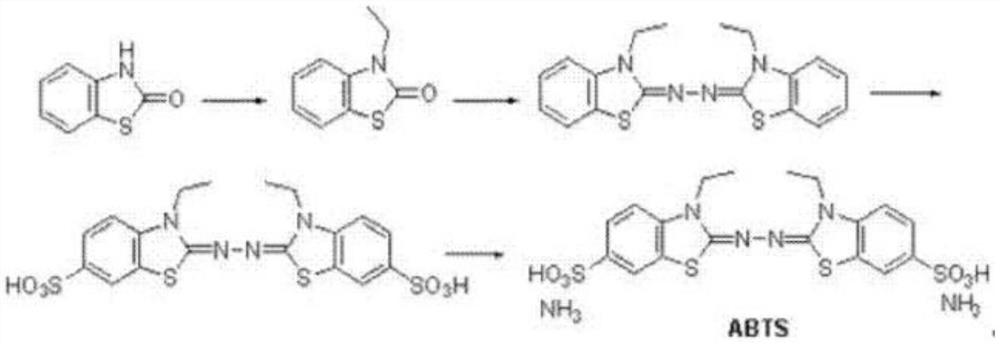
Synthetic method of 2,2'-hydrazine-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt
A technology of benzothiazoline and hydroxybenzothiazole, applied in the field of chemical synthesis, can solve the problems of high price of ABTS and limited synthesis route, and achieve the effects of low cost, high yield and excellent synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] (1) Dissolve 2-hydroxybenzothiazole (1mol, 151g), 2-bromoethane (1.1mol, 120g) and potassium hydroxide (1mol, 56g) in 2L of tetrahydrofuran, stir at 0°C React for 1 hour, then react at room temperature for 2 hours, after the reaction is complete, spin off the tetrahydrofuran solvent, add 2L of ethyl acetate, wash twice with water, dry over anhydrous sodium sulfate, then spin off the ethyl acetate, wash with methyl tert-butyl ether Crystallization gave 150 g of 3-ethylbenzothiazol-2-one, with a yield of 84%. HNMR (DMSO- d 6 ,400MHz): 7.67-7.60 (m, 1H), 7.41-7.27 (m, 2H), 7.23-7.14 (m, 1H), 3.97(q, 2H), 1.19(t,3H).
[0024] (2) Dissolve 3-ethylbenzothiazol-2-one (1mol, 179g) obtained in step (1) in 1000mL of ethanol, add 80% hydrazine hydrate (0.625mol, 31g), heat and reflux for 4 hours , TLC detection, no reaction. Then add concentrated hydrochloric acid (0.12mol, 10mL), continue to stir, gradually precipitate, continue to react for 3 hours, cool, filter with suction...
Embodiment 2
[0029] (1) Dissolve 2-hydroxybenzothiazole (1mol, 151g), 2-bromoethane (1.2mol, 131g) and sodium hydroxide (1.1mol, 44g) in 2L of acetonitrile, at 0°C , stirred and reacted for 1 hour, then reacted at room temperature for 2 hours, after the reaction was completed, the acetonitrile solvent was spun off, 2L of dichloromethane was added, washed twice with water, dried over anhydrous sodium sulfate, then the dichloromethane was spun off, and methyl tert-butyl 153 grams of 3-ethylbenzothiazol-2-one were obtained by crystallization of the base ether, and the yield was 85%.
[0030] (2) Dissolve 3-ethylbenzothiazol-2-one (1mol, 179g) obtained in step (1) in 1000mL of methanol, add 80% hydrazine hydrate (0.625mol, 31g), and then add concentrated hydrochloric acid (0.14mol, 12mL), heated and refluxed for 4 hours, the reaction was completed, cooled, suction filtered and washed with cold methanol to obtain 151 grams of 2,2'-azinobis(3-ethylbenzothiazoline), yield 85 %.
[0031] (3) Slo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com