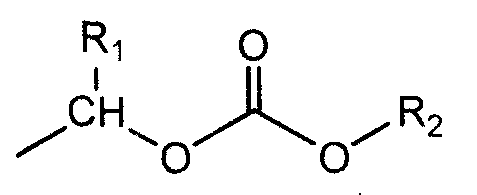
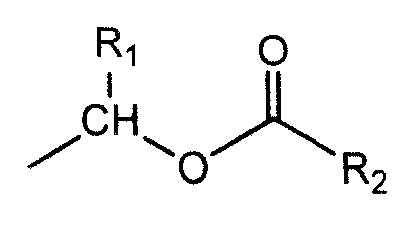
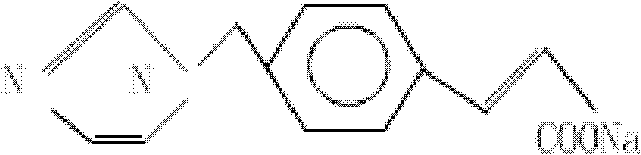
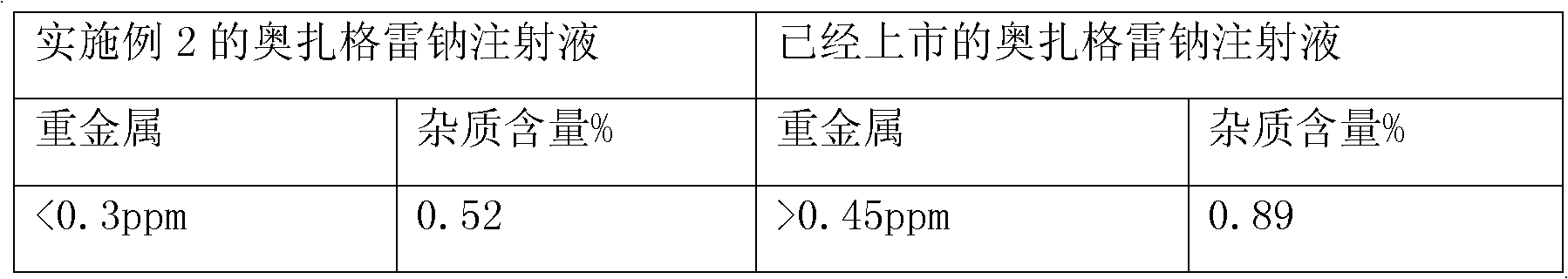
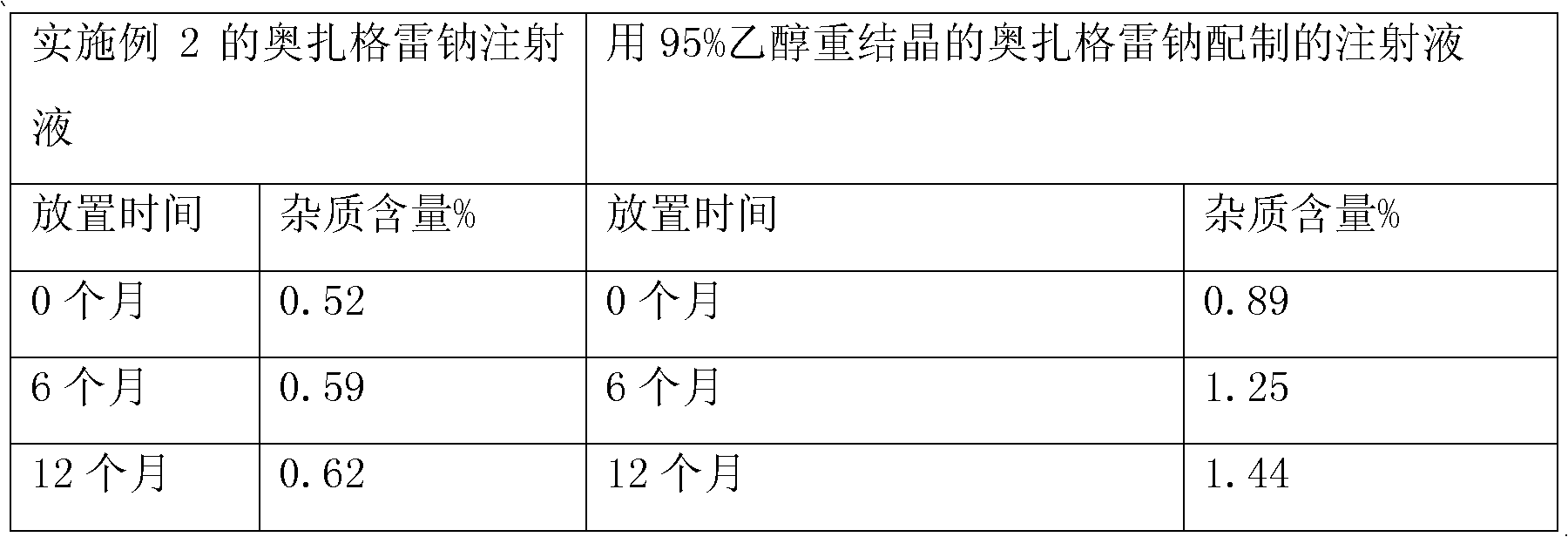
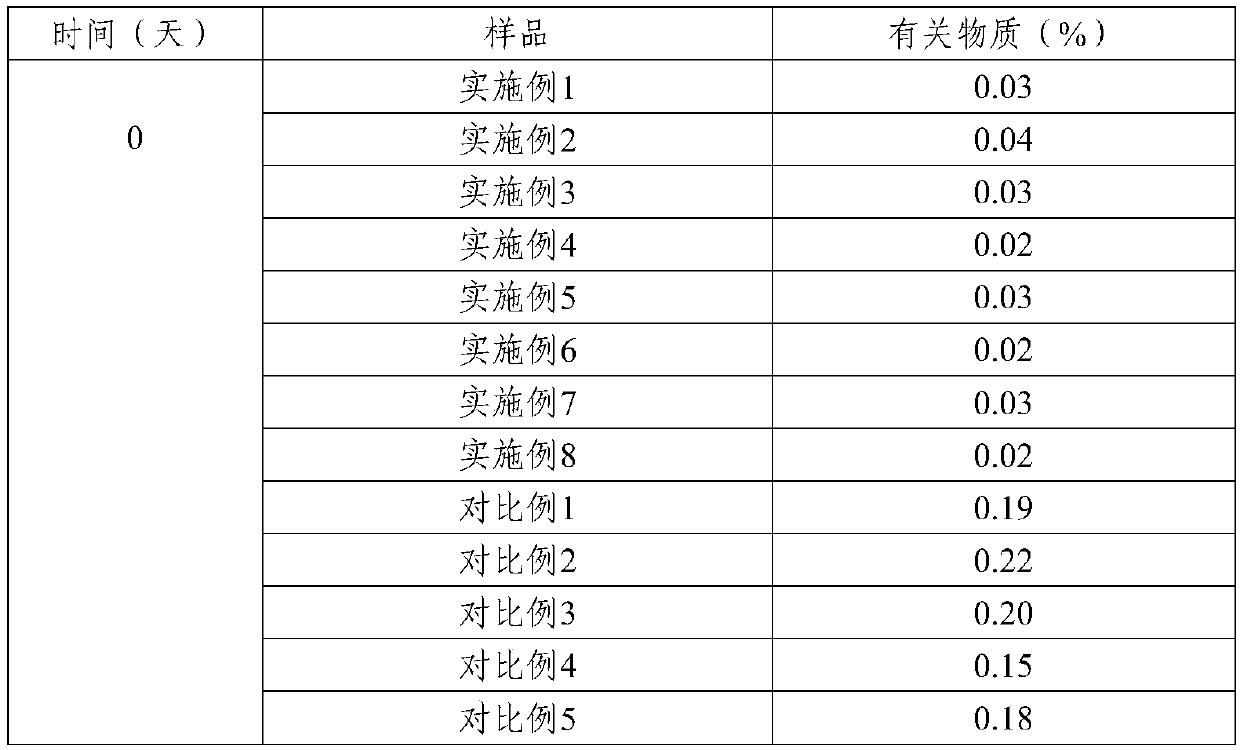
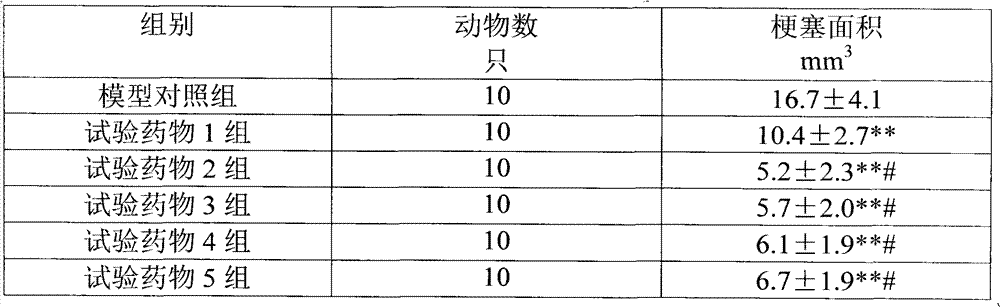
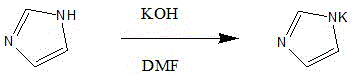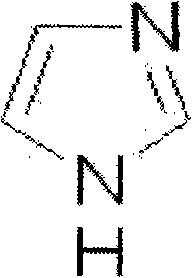Patents
Literature
52 results about "Ozagrel" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
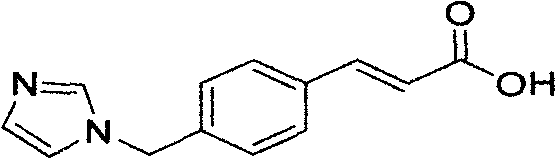
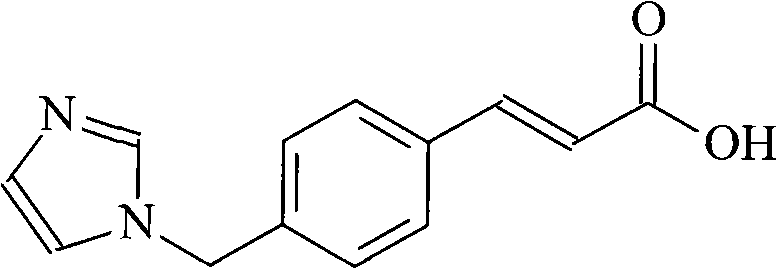
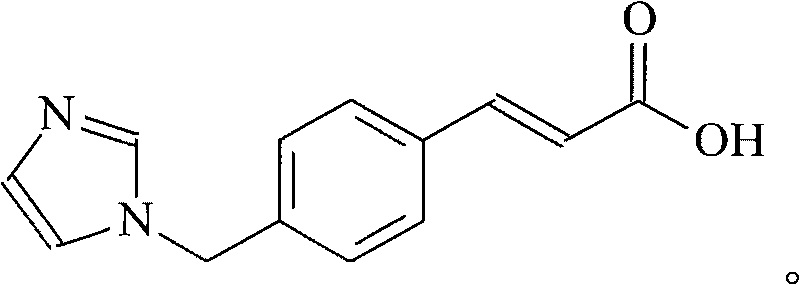
Ozagrel (INN) is an antiplatelet agent working as a thromboxane A2 synthesis inhibitor.
Ozagrel sodium injection and preparation method thereof
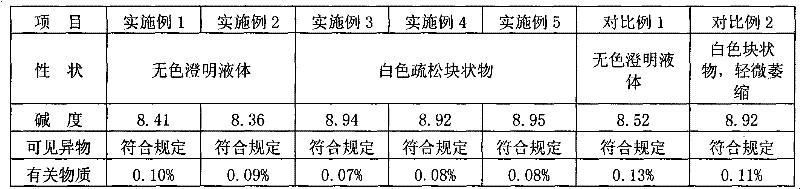
ActiveCN101695475AQuality assuranceEnsure stabilityOrganic active ingredientsPowder deliverySolventExcipient
The invention relates to an ozagrel sodium injection and a preparation method thereof, in particular to an ozagrel sodium injection for treating acute thrombotic cerebral infarction and movement disorders concomitant with cerebral infarction, preferably injection solution and lyophilized powder injection. The ozagrel sodium injection mainly comprises ozagrel serving as an active component and sodium hydroxide and citric acid serving as auxiliary materials. Solvent in the injection solution is water for injection. Excipient in the lyophilized powder injection is mannitol and / or sorbitol which can be combined in any one or two optional medicinal proportions, or no excipient is added.
Owner:HAINAN LEVTEC PHARMA
Oral enteric preparation containing Grel drugs and aspirin
The invention relates to a novel oral enteric preparation which is composed of 0.1-1000mg of Grel drugs, or medically acceptable salts, ester or derivatives, 37.5-325mg of aspirin and at least one medically acceptable load, wherein the Grel drugs, or medically acceptable salts, esters or derivatives are clopidogrel, prasugrel, brilinta, sarpogrelate, ozagrel, anagrelide, pamicogrel, or medically acceptable salts, ester or derivatives, preferably, clopidogrel sulfate. The oral enteric preparation is used for curing acute coronary syndrome (ACS), angor pectoris, stroke, myocardial infarction or cardia cerebrovascular diseases of patients. According to the oral enteric preparation, adverse reactions such as functional gastrointestinal disorders, nausea, vomit, gastritis, concealed hemorrhage, ulcer exacerbation and gastrointestinal bleeding caused by strong stimulus of aspirin to stomach can be avoided.
Owner:王定豪
Preparation method of ozagrel bulk drug
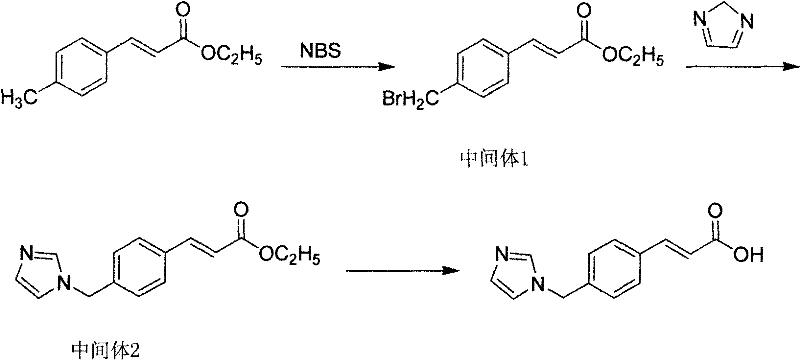
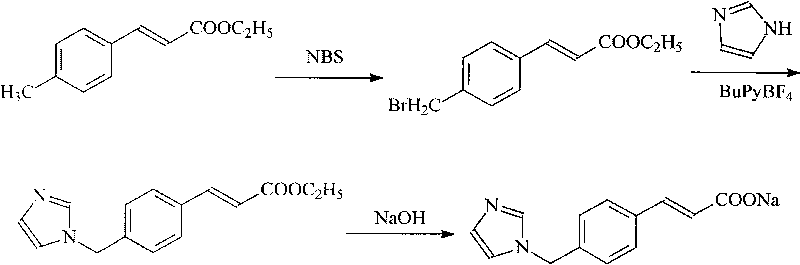
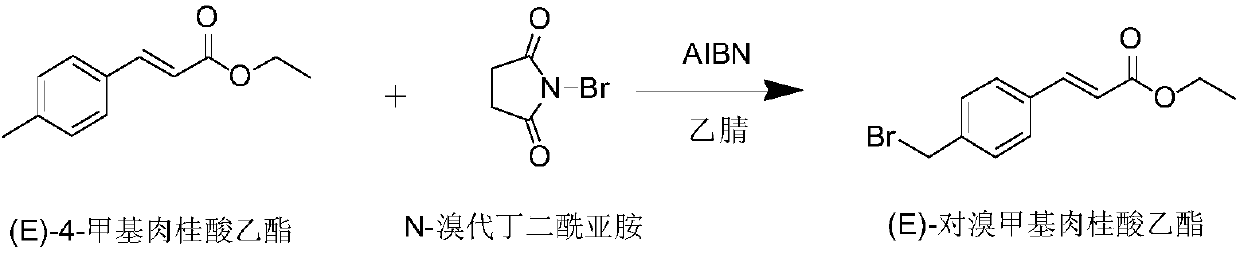
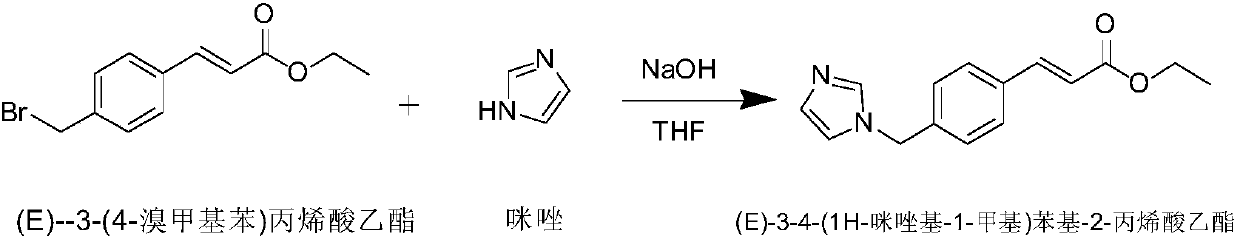
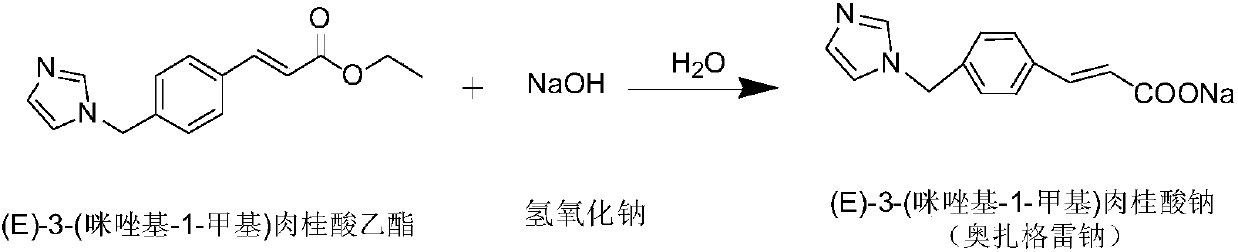
The invention discloses a preparation method of an ozagrel bulk drug. The method comprises the following steps of: bromating methyl ethyl cinnamate serving as a starting material with NBS (B-Bromosuccinimide) to obtain ethyl 4-bromomethylcinnamate; undergoing a condensation reaction on the ethyl 4-bromomethylcinnamate and imidazole to generate ozagrel ethyl ester; hydrolyzing under an alkaline condition; performing acid precipitation to obtain crude ozagrel; and refining to obtain an ozagrel bulk drug. Due to the adoption of the method, a method for refining catalysts, solvents and crude products used in each reaction step is improved, the product yield is high, the product quality is good, the use of toxic reagents and expensive reagents is avoided, little environmental pollution is caused, and the production cost is low. The method is suitable for industrial production, and is an improved and environmentally-friendly method for preparing the ozagrel bulk drug.
Owner:辽宁远大诺康医药有限公司
High-purity ozagrel compound
InactiveCN101704786AHigh purityAvoid non-conformitiesOrganic chemistryActivated carbonForeign matter
The invention relates to a high-purity ozagrel compound, and a preparation method of the ozagrel compound guarantees the quality of raw materials, and avoids the phenomenon that visible foreign matters are unqualified in the production process. The preparation method includes the following steps: (1) adding crude ozagrel in water to form aqueous dispersion; (2) then dropwise adding sodium hydroxide solution in the aqueous dispersion until the aqueous dispersion is clear to obtain clear solution; and (3) adding activated carbon in the clear solution for adsorption, conducting filtering and decarburization, adding acid in obtained filtrate to adjust pH value, precipitating solids, filtering, washing, and drying to obtain the high-purity ozagrel.
Owner:HAINAN LINGKANG PHARMA CO LTD
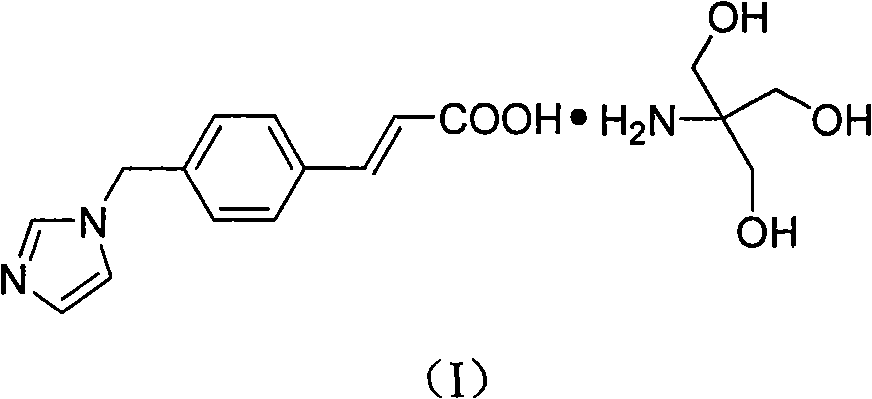
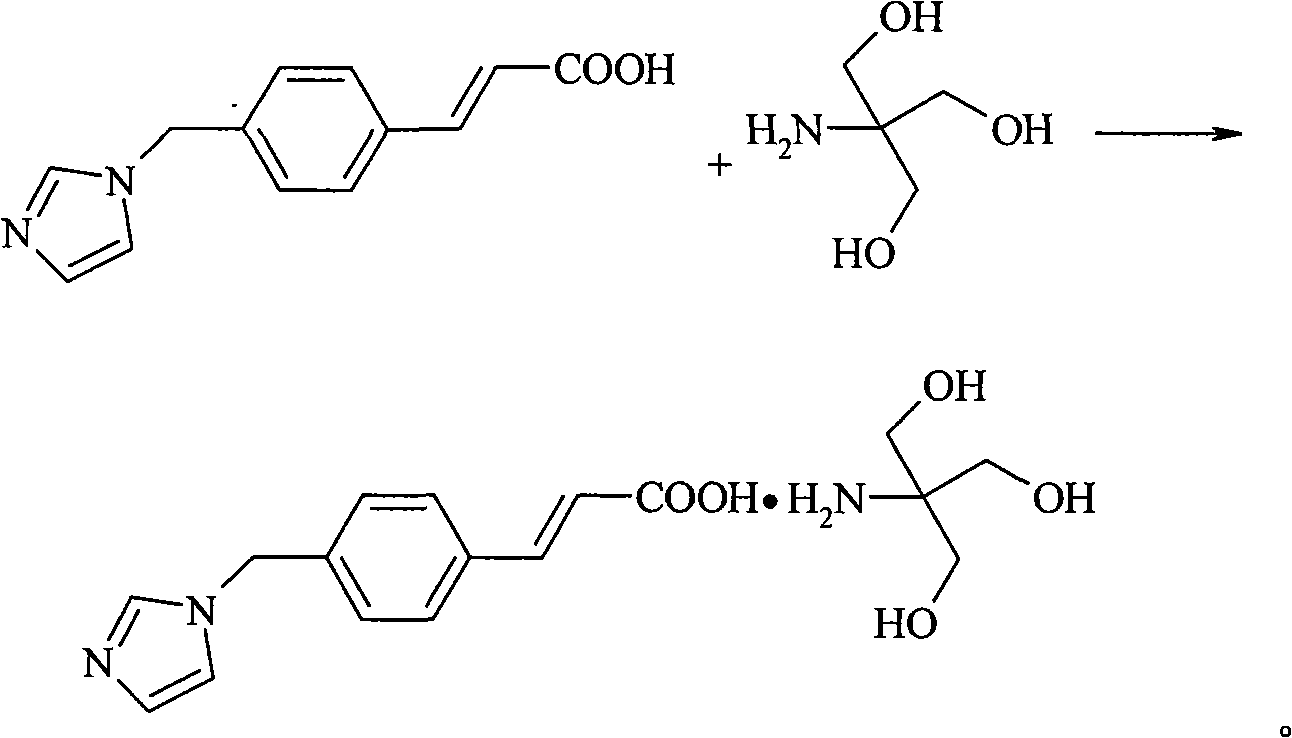
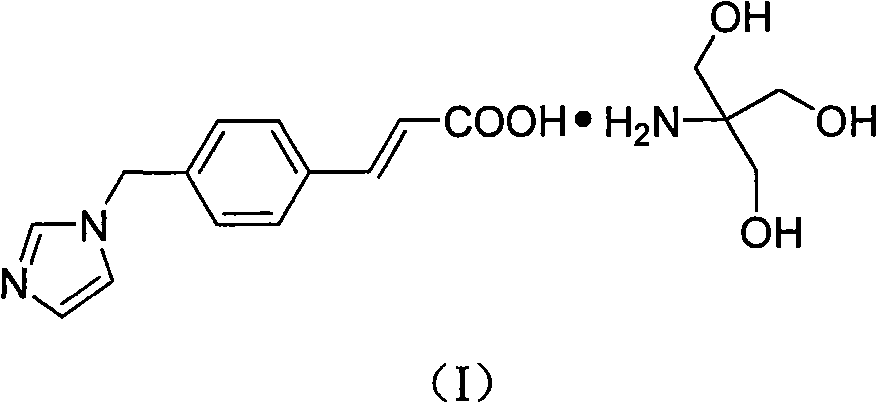
Ozagrel tromethamine, compound, preparation method and application thereof
ActiveCN101659640AGood water solubilityExcellent long-term storage stabilityOrganic active ingredientsOrganic chemistryConvulsionSolubility
The invention provides an ozagrel salt which is stable, has functions of inhibiting platelet aggregation and removing blood-vessel convulsion, has good water solubility, and is prepared into a compound suitable for clinical application. The new compound is ozagrel tromethamine; and the ozagrel and the tromethamine are prepared by reaction in a solvent. The compound has good water solubility, strong and long-term stability, and the functions of inhibiting platelet aggregation and removing blood-vessel convulsion.
Owner:徐华
Preparation method of Ozagrel sodium
The invention discloses a preparation method of Ozagrel sodium. The method comprises the following steps: performing a bromination reaction on ethyl 4-methylcinnamate and N-bromo-succinimide by takingacetonitrile as a solvent under the triggering of azodiisobutyronitrile, so as to obtain ethyl 4-bromomethylcinnamate; performing a condensation cyclization reaction on the ethyl 4-bromomethylcinnamate and imidazole by taking sodium hydroxide as an acid-binding agent and taking tetrahydrofuran as a solvent, so as to obtain imidazole ethyl 4-methylcinnamate; performing alkali hydrolysis on the imidazole ethyl 4-methylcinnamate, so as to obtain the Ozagrel sodium. According to the preparation method, the condensation cyclization reaction is performed by taking the sodium hydroxide as the acid-binding agent and taking the tetrahydrofuran as the solvent, so that the finally obtained product does not contain toxic components, the yield and the purity of the product can be effectively improved,and the content of genotoxic impurities (the ethyl 4-bromomethylcinnamate and ethyl 4-dibromomethylcinnamate) in the product is zero.
Owner:浙江科瑞医药科技有限公司
Method for preparing ozagrel intermediate (E)-4-(methyl imidazolyl) methyl cinnamate
The invention discloses a method for preparing an ozagrel intermediate (E)-4-(methyl imidazolyl) methyl cinnamate, which comprises the following steps of: (1) sodium imidazolide preparation: (1.1) dissolving sodium hydroxides in deionized water, and after the sodium hydroxides is completely dissolved, stirring the obtained product and adding imidazolide to the obtained product; (1.2) putting the solution obtained in the step (1.1) into a reaction kettle and uniformly mixing to obtain a stand-by material; and (1.3) adding toluene to the stand-by material obtained in the step (1.2), then adding acetone to the obtained mixture to obtain an acetone solution; and (2) (E)-4-(imidazolyl-methyl) methyl cinnamate preparation: (2.1) adding methyl 3-(4-bromomethyl) cinnamate to the acetone solution, and after reaction, carrying out depressurized evaporation on the obtained product to remove acetone from the material; and (2.2) adding deionized water to the material subjected to acetone evaporation in the step (2.1), and stirring the obtained mixture to crystallize, thereby obtaining the (E)-4-(imidazolyl-methyl) methyl cinnamate. By using the method disclosed by the invention, the shortages existing in the prior art can be overcome, and the production cost of the intermediate is greatly reduced.
Owner:SHANDONG CHENGCHUANG BLUE OCEAN PHARM TECH CO LTD
Ozagrel ester, composition and production method thereof
InactiveCN101219994AHigh dissolution rateImprove stabilityOrganic active ingredientsOrganic chemistrySolubilityFreeze-drying
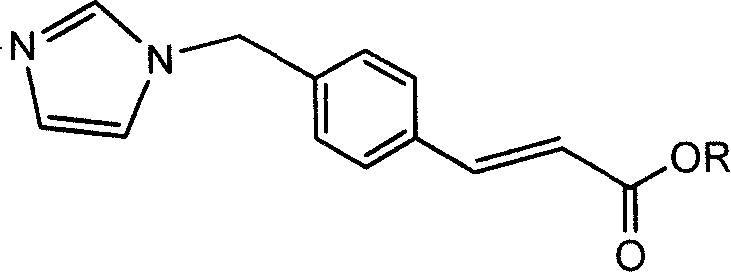
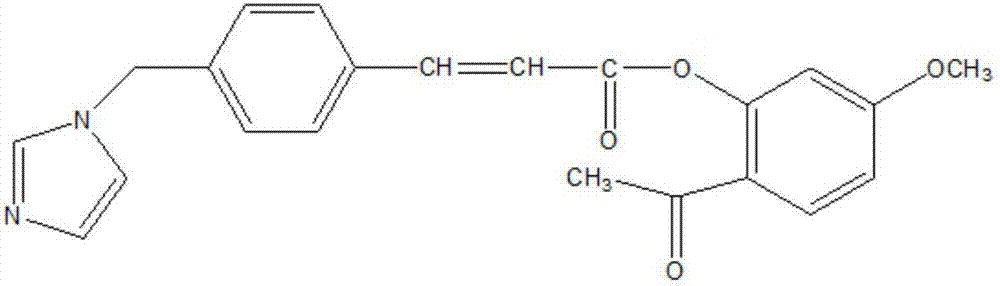
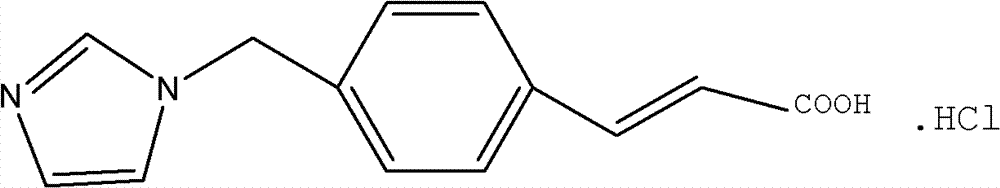
The invention discloses an ozagrel ester with the compounds of the ozagrel ester. The ozagrel ester is provided with a chemical structural formula as listed on the right. The compounds of the ozagrel ester comprise an ozagrel ester with the amount of effective treatment and a carrier accepted in medicine. The invention also discloses the preparation methods for the ozagrel ester and the compounds of the ozagrel ester. The ozagrel ester of the invention has good solubility and stability; besides, the compounds of ozagrel ester can be conveniently made into a plurality of liquid, solid, freeze-drying and mucosa or the skin medication agents.
Owner:王立峰
A more stable sodium ozagrel compound and pharmaceutical composition thereof
InactiveCN102276532AEasy to manufacturePreparation belongs to process improvementPowder deliveryOrganic active ingredientsSolventEthyl acetate
The present invention relates to a more stable sodium ozagrel compound and a pharmaceutical composition, which comprises recrystallizing the crude product sodium ozagrel with ethyl acetate:methanol=1:1 as a solvent for 1-3 times, and simultaneously using activated carbon White crystals are obtained after decolorization.
Owner:贺金凤
Ozagrel sodium drug combination for injection
InactiveCN102846561AFix stability issuesHigh yieldPowder deliveryOrganic active ingredientsProduction rateMANNITOL/SORBITOL
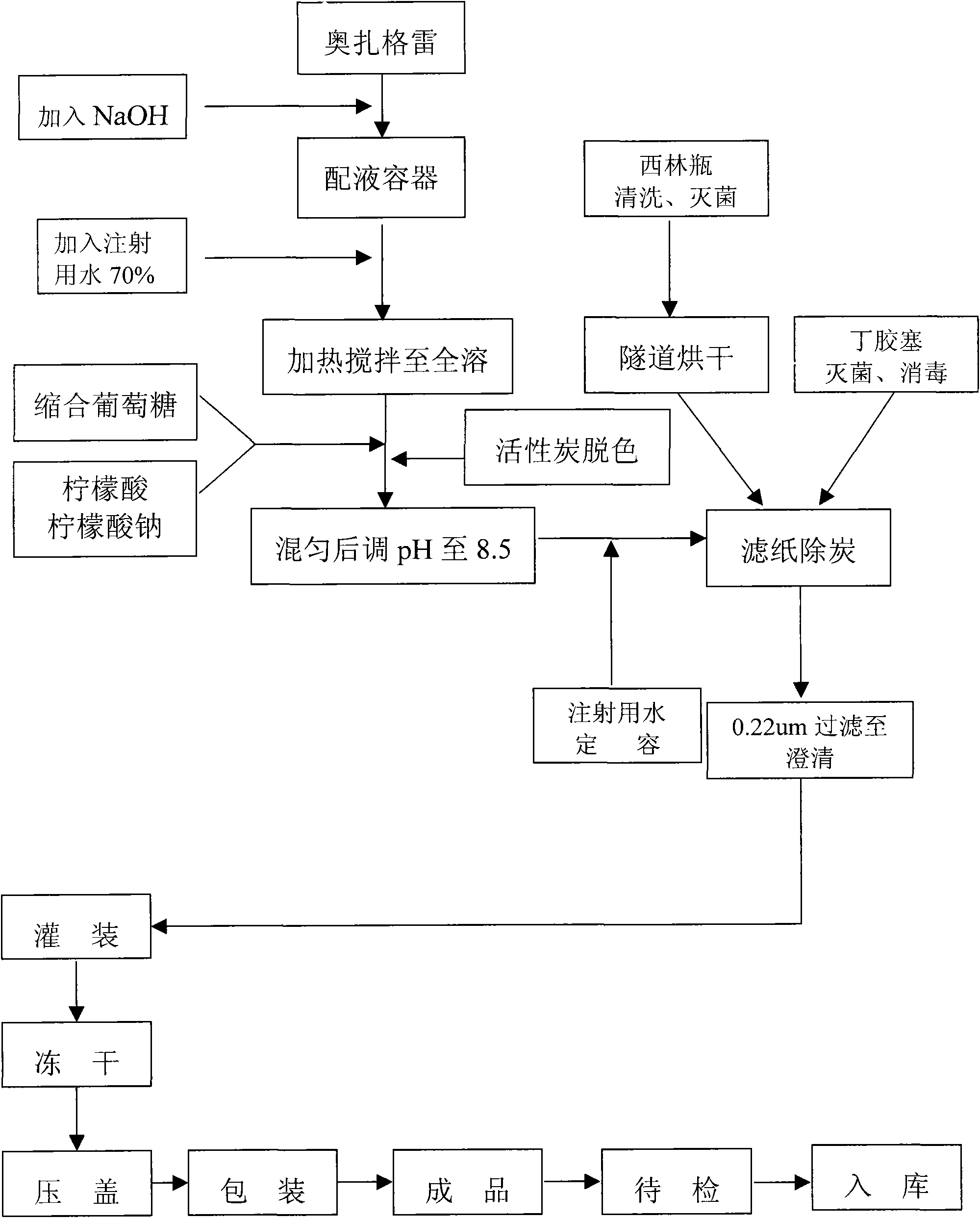
The invention discloses an ozagrel sodium drug combination for injection. The ozagrel sodium injection solution consists of ozagrel sodium, mannitol and anhydrous sodium carbonate, wherein each piece contains 20-80 mg of ozagrel sodium, 20-80 mg of mannitol and 5-15 mg of anhydrous sodium carbonate. The drug combination is prepared by a method comprising the following steps: taking a recipe quantity of injection water, adding the mannitol and the anhydrous sodium carbonate and stirring until the mannitol and the anhydrous sodium carbonate are dissolved; adding the ozagrel sodium, and stirring until the ozagrel sodium is fully dissolved; regulating a pH value between 7.7 and 8.7; adding medicinal carbon and stirring; leaching, replenishing the injection water to a full quantity and uniformly mixing; finely filtering; encapsulating; freeze-drying; inspecting with a light; and warehousing to obtain the ozagrel sodium drug combination. The ozagrel sodium drug combination has good stability. The production rate of the product is increased, the cost is lowered, and industrialization is realized. The drug combination can be better applied in clinic and has more remarkable advantages.
Owner:TIANJIN SONGRUI MEDICAL TECH
Ozagrel compound, preparation method and pharmaceutical composition of ozagrel compound
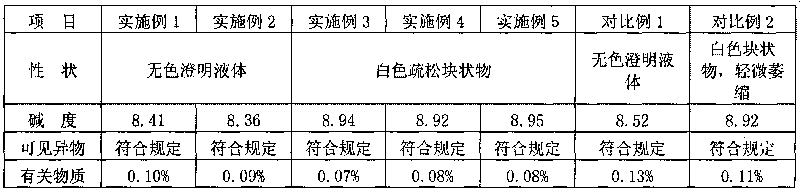
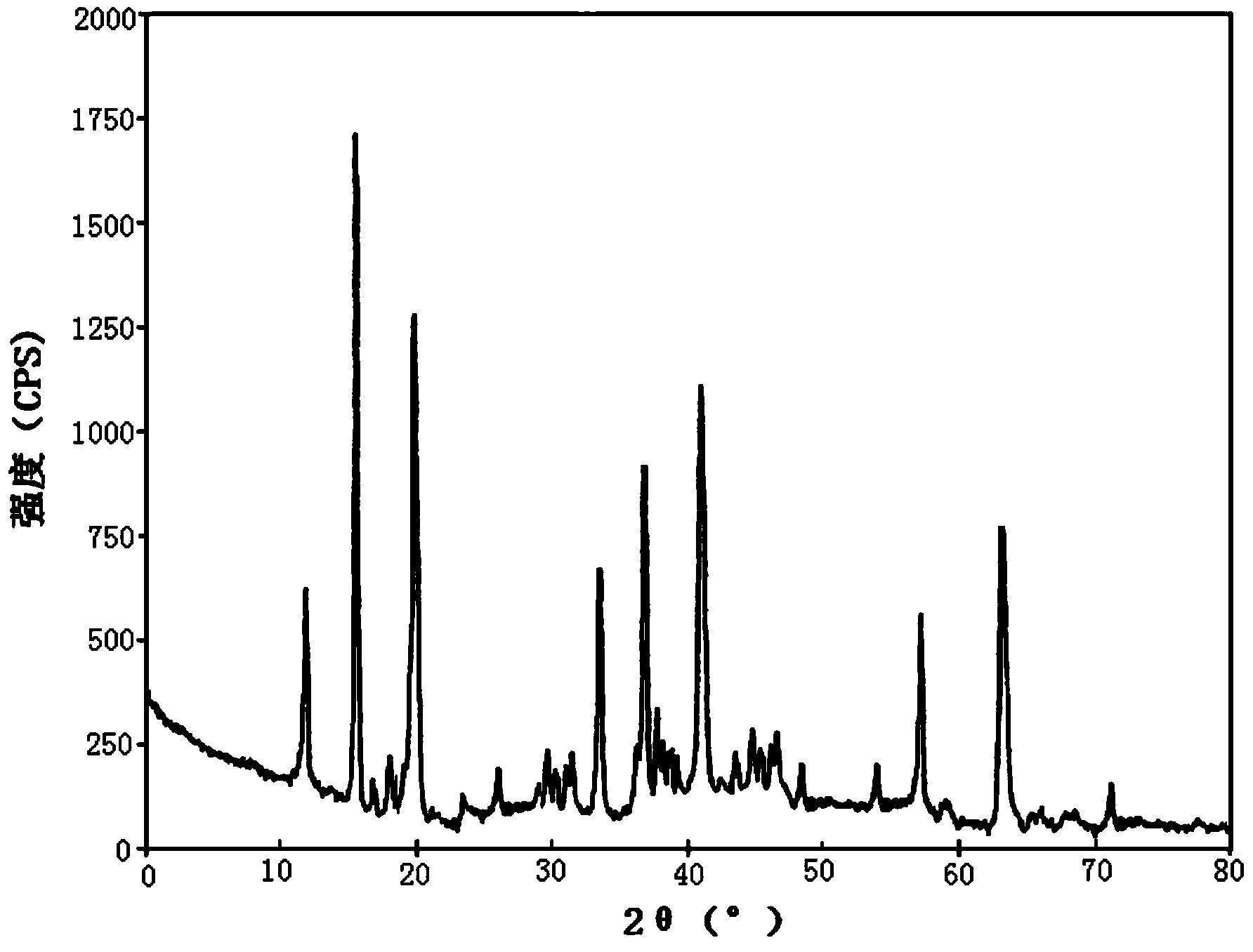
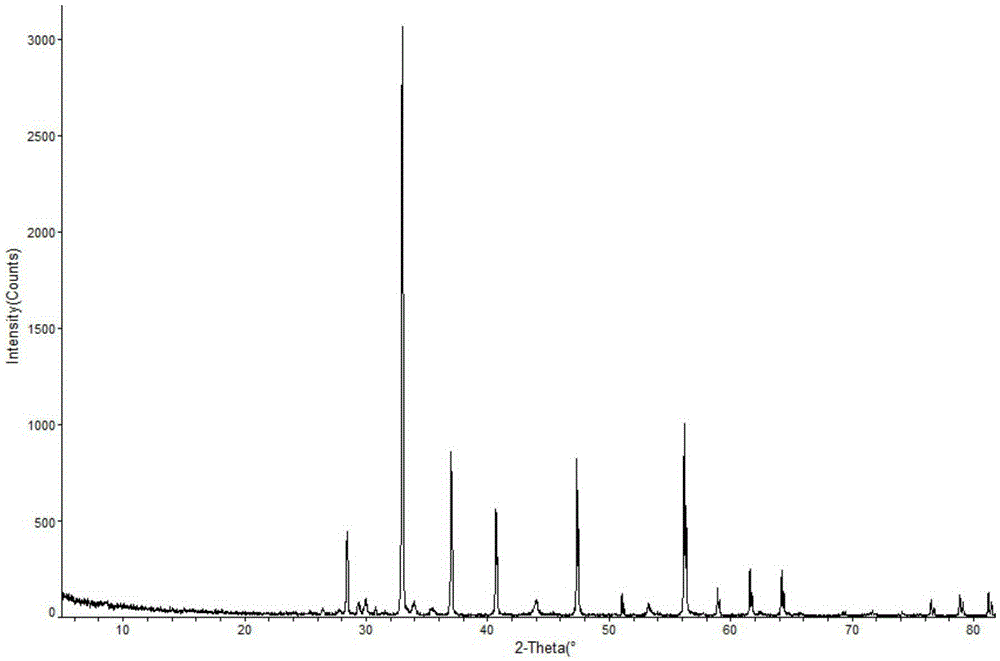
ActiveCN103450086ALittle changeReduce contentOrganic active ingredientsPowder deliveryCombinatorial chemistryOzagrel
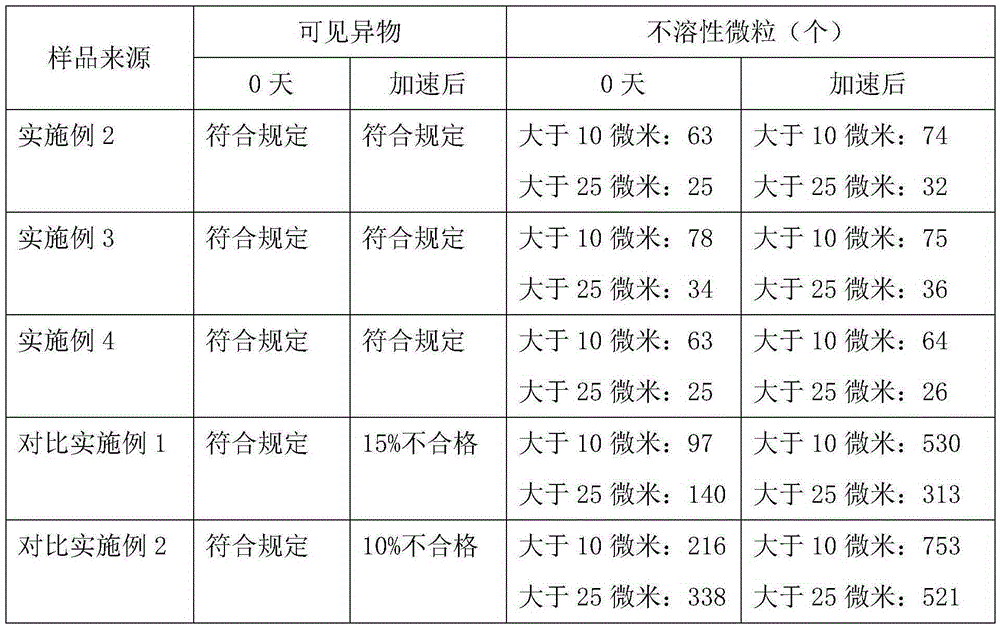
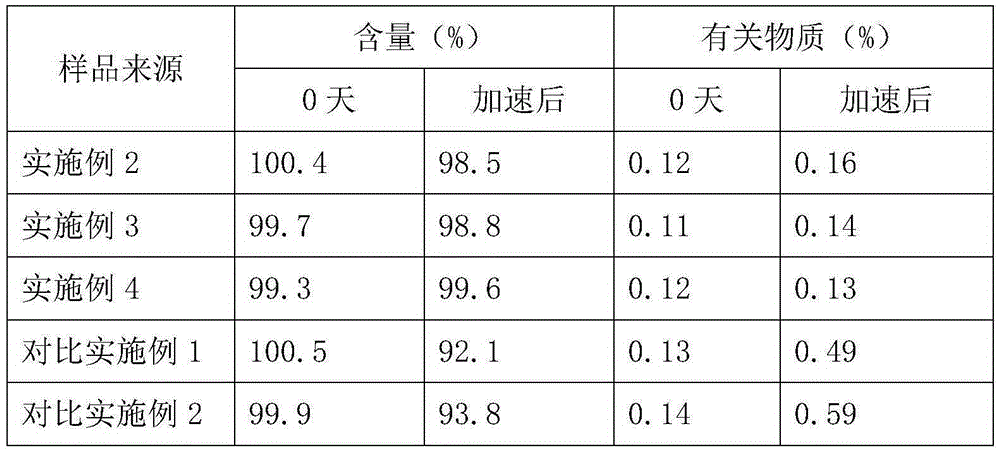
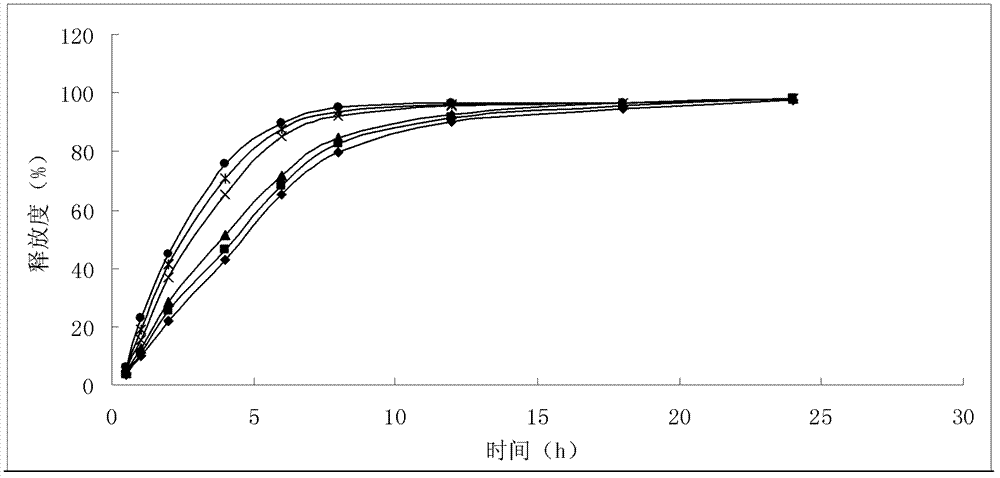
The invention belongs to the technical field of medicines, and particularly relates to an ozagrel compound, a preparation method and a pharmaceutical composition of the ozagrel compound. The X-ray powder diffraction spectrogram obtained by Cu-K alpha-ray measurement is shown in a figure 1; and the structural formula is as shown in a formula (I). The ozagrel compound is a novel crystal form which is different from that in the prior art. The novel crystal form ozagrel compound has humidity, temperature and illumination stability obviously superior to those of the ozagrel in the prior art; sodium ozagrel for injection, prepared from the ozagrel compound of such crystal form, is high in redissolving performance, and insoluble particles have smaller change after an injection solution is combined; after redissolving, the contents of specific impurities I and II in the solution are obviously less than those in the prior art.
Owner:北京科创鼎诚医药科技有限公司
Sodium ozagrel freeze-dried powder for injection and preparation method of sodium ozagrel freeze-dried powder
InactiveCN104352451AImprove qualityImprove stabilityOrganic active ingredientsPowder deliverySide effectMANNITOL/SORBITOL
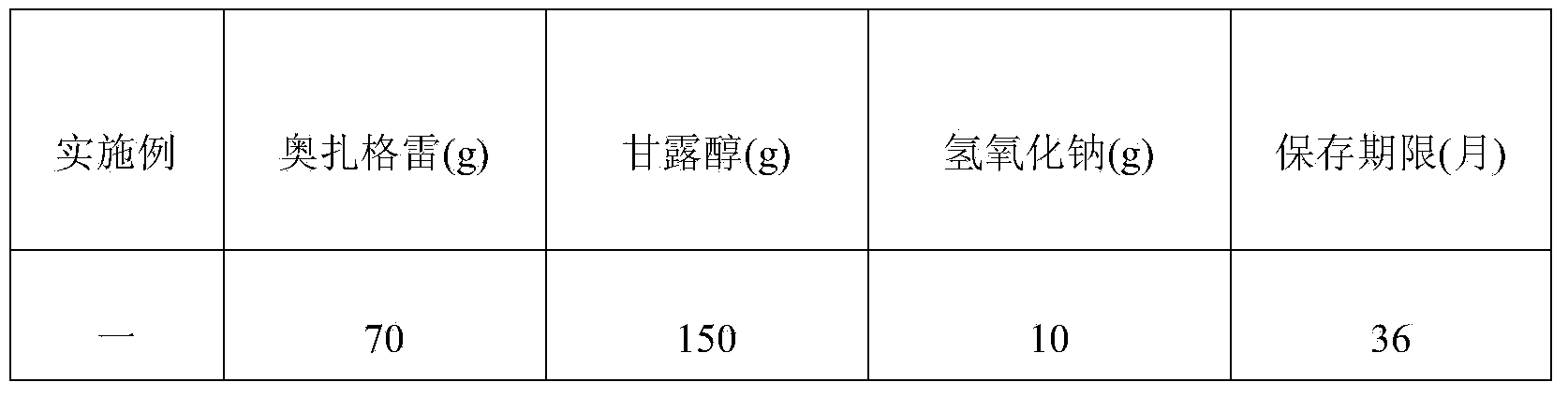
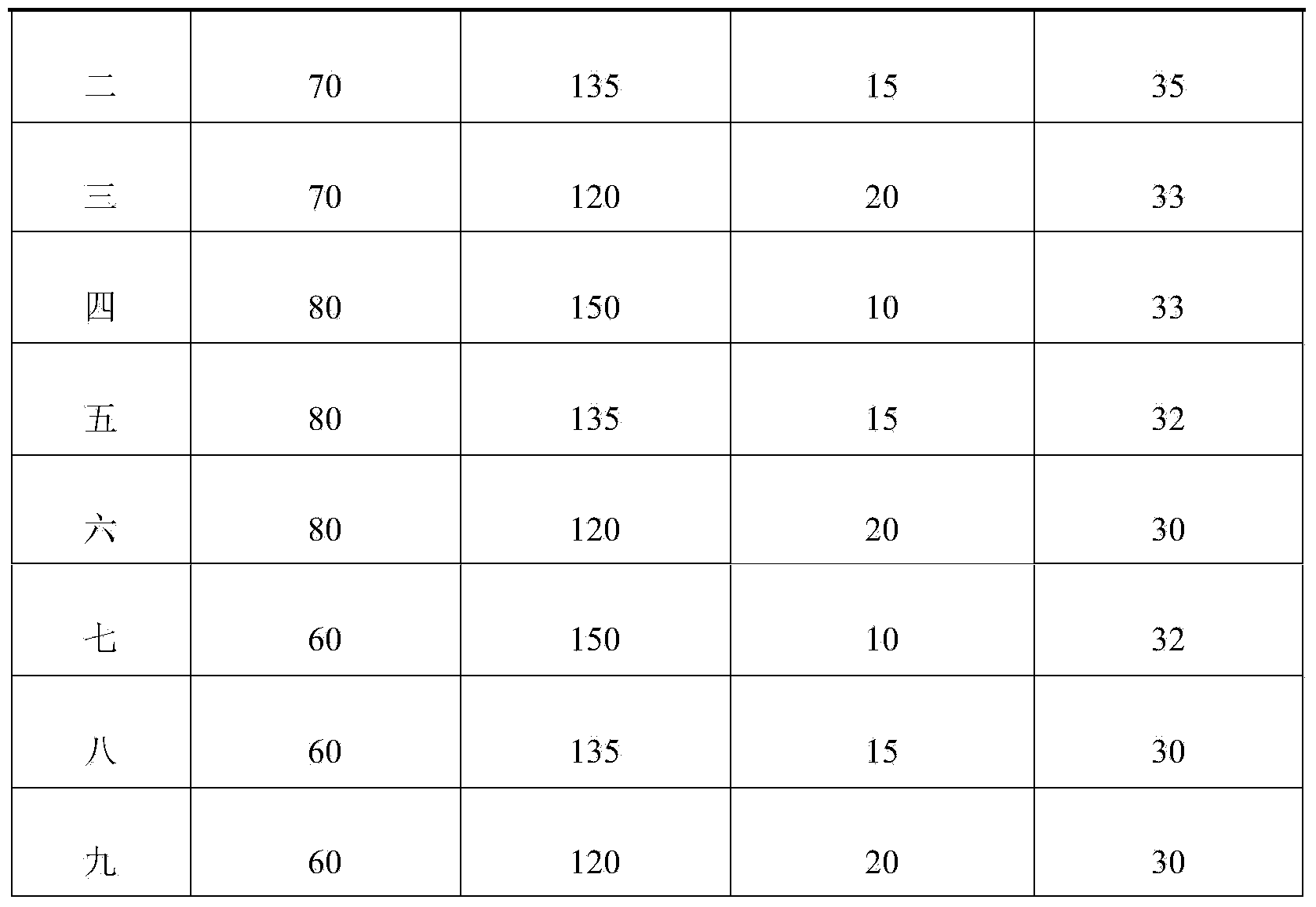
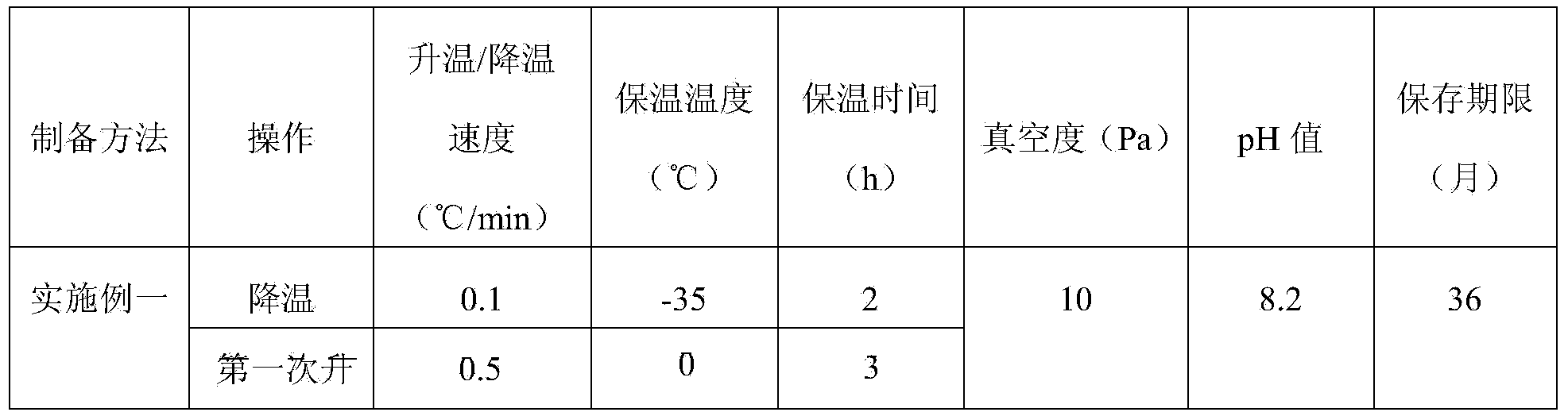
The invention provides sodium ozagrel freeze-dried powder for injection. The sodium ozagrel freeze-dried powder comprises formula components in parts by weight as follows: 60-80 parts of ozagrel, 120-150 parts of mannitol and 10-20 parts of sodium hydroxide. The sodium ozagrel freeze-dried powder has simple formula and fewer auxiliary materials and is applicable to popularization, side effects caused by excessive addition of auxiliary materials are avoided, and the safety of clinical use is improved. The invention further provides a preparation method of the sodium ozagrel freeze-dried powder for injection. Through accurate control on parameters such as the heating speed, the holding temperature, the holding time, the pressure and the like of freeze-drying process steps, the obtained sodium ozagrel freeze-dried powder for injection is more normative in preparation course, good in product quality and high in stability.
Owner:HAINAN GENERAL & KANGLI PHARMA
Method for preparing paeonol-ozagrel conjugate lipidosome through ethanol injection method
InactiveCN106943352AImprove bioavailabilityImprove stabilityKetone active ingredientsPharmaceutical non-active ingredientsCholesterolPhosphate
The invention discloses a method for preparing a paeonol-ozagrel conjugate lipidosome through an ethanol injection method. The paeonol-ozagrel conjugate lipidosome is prepared from a paeonol-ozagrel conjugate, a phospholipid material, cholesterol, an inorganic solvent and a buffer solution. The method comprises the steps of stirring a prescription dosage of phosphate buffer solution and setting the temperature to 40 DEG C; weighing a prescription dosage of paeonol-ozagrel conjugate, soybean lecithin and cholesterol into the absolute ethyl alcohol for ultrasonic treatment for 4min, slowly adding the drug to the phosphate buffer solution by using a 1mL injector at a constant speed, further stirring for two hours and granulating the finally obtained suspension through a 0.80micron microfiltration membrane to obtain the paeonol-ozagrel conjugate lipidosome. According to the method, the bioavailability and the stability of a paeonol-ozagrel conjugate are improved, thrill is reduced and the drug action time is prolonged.
Owner:上海海虹实业(集团)巢湖今辰药业有限公司
New preparation method of Ozagrel sodium powder injection
InactiveCN101596167ALittle side effectsConvenient for clinical operationOrganic active ingredientsPowder deliverySide effectCLARITY
The invention researches a new preparation method by aiming at the problems of side effect and inconvenient use of the clinical application of an Ozagrel sodium refrigerated dry powder injection and applies polyglucose to the Ozagrel sodium refrigerated dry powder injection for the first time, the Ozagrel sodium refrigerated dry powder injection is decolorized by active carbon and filtered by a filter film with the aperture of 0.22 micrometer so as to solve the poor appearance problem of the Ozagrel sodium powder injection, achieve the purposes of convenient use, cost reduction, curative effect guarantee and side effect reduction and solve the problem of unqualified clarity generated by applying the polyglucose to the refrigerated dry injection.
Owner:HARBIN PHARMA GROUP BIOLOGICAL ENG
Injection ozagrel sodium freeze-dried powder for treating cerebral infarction
InactiveCN105267162AImprove stabilityReduce typesOrganic active ingredientsPowder deliveryFreeze-dryingMicroparticle
The invention discloses injection ozagrel sodium freeze-dried powder for treating cerebral infarction, and belongs to the technical field of medicines. The ozagrel sodium is a crystal, and the novel crystal form of ozagrel sodium, provided by the invention, is different from a crystal structure of the prior art, and experiments prove that the novel crystal form compound is high in purity, good in fluidity and stability and low in impurity content, cannot absorb moisture easily, is safe and reliable in clinical application; and a powder injection prepared by using the novel crystal form compound is good in stability after being compatible with a solvent and extremely low in insoluble micro-particle content, and is very suitable for clinical application.
Owner:南京多宝生物科技有限公司
Method for preparing sodium ozagrel freeze-dried powder injection
ActiveCN105287408AControl contentImprove quality stabilityOrganic active ingredientsPowder deliveryFreeze-dryingOzagrel
The invention provides a method for preparing a sodium ozagrel freeze-dried powder injection. The method comprises the following steps: a) mixing ozagrel, a sodion-containing alkaline matter and first injection water, and conducting pH value adjustment and adsorption treatment in sequence to obtain a mixed solution A; b) mixing the mixed solution A with second injection water, and conducting refrigeration and drying in sequence so as to obtain the sodium ozagrel freeze-dried powder injection; the volume ratio of the first injection water to the second injection water is 6:(3-5). Compared with the prior art, the provided preparation method can effectively control the content of impurities in the sodium ozagrel freeze-dried powder injection, and can improve the quality stability of the product. Experiment results show that the content of the impurities in the sodium ozagrel freeze-dried powder injection obtained by the provided preparation method is 0.3% or below.
Owner:HUNAN KELUN PHARMA
Medicinal composition, preparation method and quality control method
InactiveCN1915250AAvoid disadvantagesGood curative effectOrganic active ingredientsPharmaceutical delivery mechanismDiseaseCoronary artery disease
A composite medicine in the form of injection or orally taken medicine for treating cardiovascular and cerebrovascular diseases, such as coronary heart disease, angina pectoris, apoplexy sequelae, cerebral thrombus, etc, is prepared from Chinese angelica root or its extract and ozagrel. Its preparing process and quality control method are also disclosed.
Owner:BEIJING QI YUAN YI DE PHARMA RESEARCH CENTER
Medicinal composition, preparation method and quality control method
InactiveCN1915259AAvoid disadvantagesGood curative effectOrganic active ingredientsPharmaceutical delivery mechanismDiseaseCoronary artery disease
A composite medicine in the form of injection or orally taken medicine for treating cardiovascular and cerebrovascular diseases, such as coronary heart disease, angina pectoris, apoplexy sequelae, cerebral thrombus, etc, is prepared from acanthopanax bark or its extract and ozagrel. Its preparing process and quality control method are also disclosed.
Owner:BEIJING QI YUAN YI DE PHARMA RESEARCH CENTER
Preparation process of ozagrel intermediate methyl (E)-4-(imidazolylmethyl) cinnamate
The invention discloses a preparation process of an ozagrel intermediate methyl (E)-4-(imidazolylmethyl) cinnamate. The preparation process comprises the following steps: (1) preparing imidazolium salt: putting DMF (dimethyl formamide) in a reaction kettle, putting potassium hydroxide and imidazole, stirring the materials to react, then adding potassium carbonate, stirring the materials for hours and removing water generated by a reaction system, thus obtaining a DMF solution of imidazolium salt; (2) preparing methyl (E)-4-(imidazolylmethyl) cinnamate: dissolving methyl p-bromomethyl cinnamate in DMF and dropwise adding the solution to the DMF solution of imidazolium salt; adding deionized water and stirring the materials for crystallization, thus obtaining methyl (E)-4-(imidazolylmethyl) cinnamate. The preparation process has the beneficial effects that the appearance of obtained methyl (E)-4-(imidazolylmethyl) cinnamate is obviously changed and methyl (E)-4-(imidazolylmethyl) cinnamate becomes an off-white solid, thus being beneficial to improving the quality of ozagrel; the production cycle is substantially shortened and the material cost is reduced.
Owner:DEZHOU HANHUA CHEM
Medicinal composition, preparation method and quality control method
InactiveCN1915256ALong-term useAvoid disadvantagesOrganic active ingredientsPharmaceutical delivery mechanismDiseaseCoronary artery disease
A composite medicine in the form of injection or orally taken medicine for treating cardiovascular and cerebrovascular diseases, such as coronary heart disease, angina pectoris, apoplexy sequelae, cerebral thrombus, etc, is prepared from Chuan-xiong rhizonme or its extract and ozagrel. Its preparing process and quality control method are also disclosed.
Owner:BEIJING QI YUAN YI DE PHARMA RESEARCH CENTER
Method for preparing ozagrel sodium crystal
ActiveCN101397272BHigh purityHigh yieldOrganic chemistryCardiovascular disorderMicrofiltration membraneTherapeutic effect
The invention relates to a simple technical process used for preparing high-pure ozagrel sodium crystal. The process basically includes the following steps: the raw ozagrel sodium is added into ethanol and then heated for dissolving; active carbon is added for decoloring and filtering is carried out under the heated condition; the filtrate is filtered in a refined way by a microfiltration membrane, collected, heated first and then cooled slowerly; and the temperature is controlled to be 25 DEG C to 60 DEG C for temperature-preservation crystallization and then the temperature is reduced slowly and cooled for crystallization; finally the crystal is collected. The crystal prepared by the method is high in purity and yielding and low in impurity content; therefore the therapy effect of the product is effectively enhanced and the cost is reduced simultaneously.
Owner:HAINAN BIKAI PHARM CO LTD
Therapeutic agent for dry eye syndrome accompanied by Sjogren's syndrome
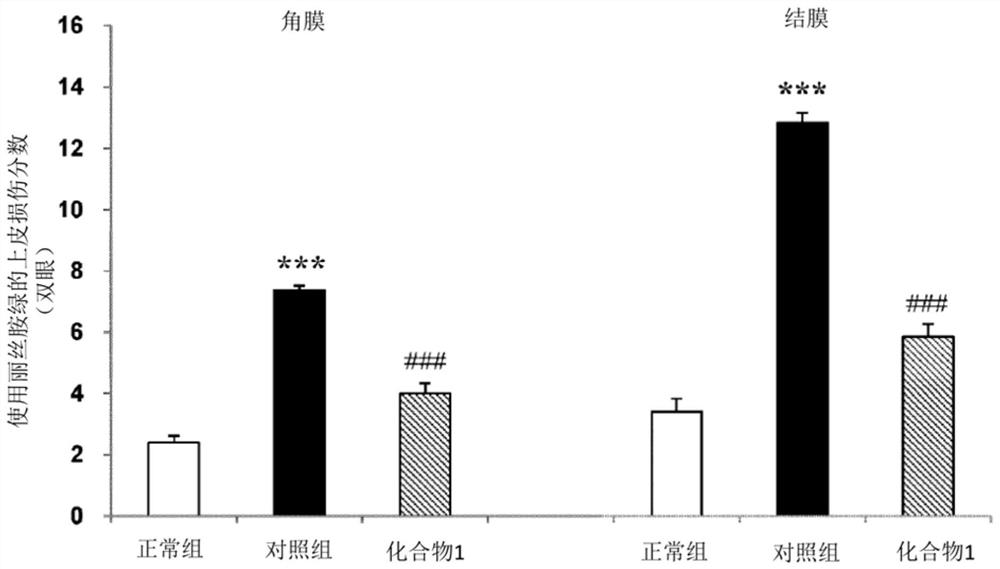
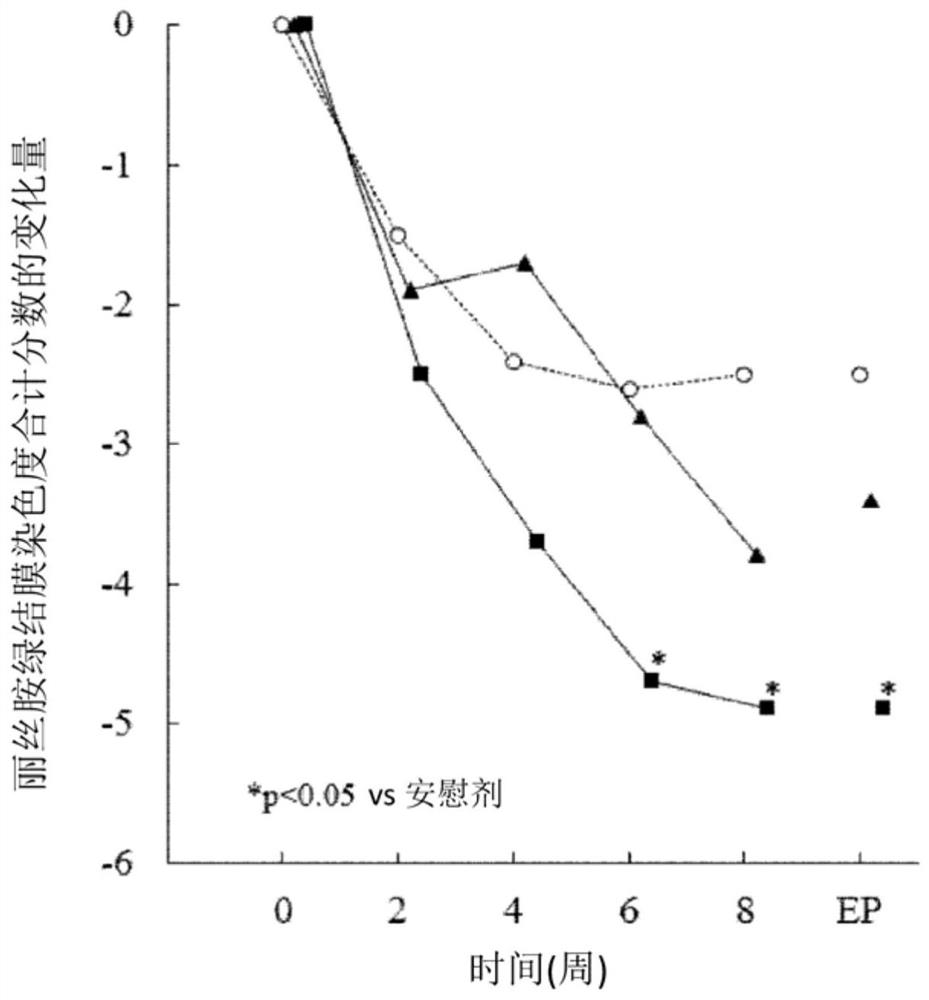
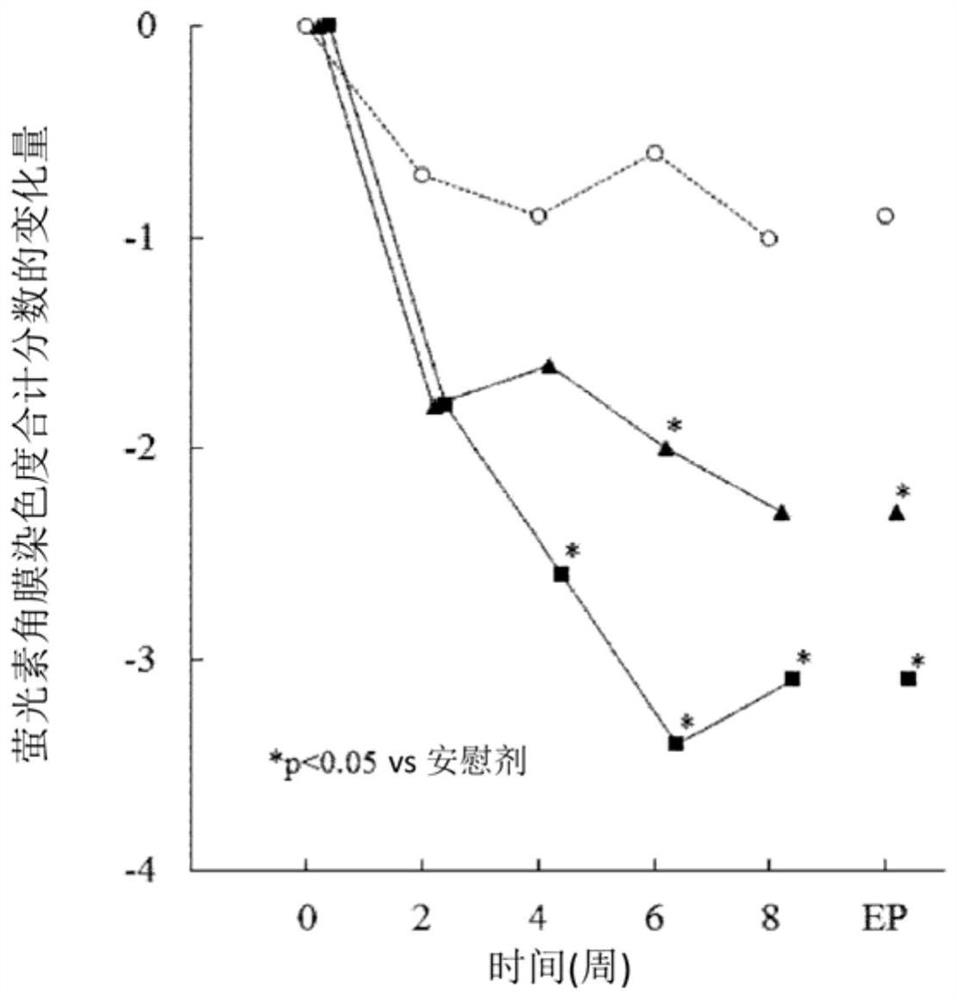
The present invention relates to a pharmaceutical composition for treating dry eye associated with Sjögren's syndrome, containing Ozagrel or a pharmacologically acceptable salt thereof as an active ingredient. The pharmaceutical composition of the present invention plays an important role in the treatment of corneal and conjunctival epithelial injuries associated with dry eye syndrome of Sjögren's syndrome, which still tends to remain strongly after dripping general dry eye syndrome therapeutic drugs (such as artificial tears, etc.). Especially excellent therapeutic effect. Therefore, the pharmaceutical composition of the present invention is useful as a therapeutic pharmaceutical composition for dry eye syndrome accompanied by Sjogren's syndrome.
Owner:TEIKA PHARMA CO LTD
(e)-4-(1-imidazole methyl) the preparation method of methyl cinnamate
The invention belongs to the medicine technical field, and in particular, relates to a preparation method of an ozagrel intermediate namely (E)-4-(1-imidazole methyl)methyl cinnamate, wherein the method includes the following steps: (a) preparation of imidazole negative ions, and (b) preparation of (E)-4-(1-imidazole methyl)methyl cinnamate; the method has the advantages of high yield and less impurity.
Owner:山东致泰医药技术有限公司
Method for preventing and treating thrombogenesis
The invention relates to a method for preventing and treating thrombogenesis. The method comprises the step of supplying Ozagrel hydrochloride oral preparation to an object needing prevention and treating of thrombogenesis, with 25-800mg and 1-4 times each day.
Owner:FUKANGREN BIO PHARMA
Ozagrel sodium injection and preparation method thereof
ActiveCN101695475BQuality assuranceEnsure stabilityPowder deliveryOrganic active ingredientsMannitolPharmaceutical Aids
The invention relates to an ozagrel sodium injection and a preparation method thereof, in particular to an ozagrel sodium injection for treating acute thrombotic cerebral infarction and movement disorders concomitant with cerebral infarction, preferably injection solution and lyophilized powder injection. The ozagrel sodium injection mainly comprises ozagrel serving as an active component and sodium hydroxide and citric acid serving as auxiliary materials. Solvent in the injection solution is water for injection. Excipient in the lyophilized powder injection is mannitol and / or sorbitol which can be combined in any one or two optional medicinal proportions, or no excipient is added.
Owner:HAINAN LEVTEC PHARMA
Liposome solid preparation of ozagrel
InactiveCN102626388BImprove product qualityUniform particle sizeOrganic active ingredientsPharmaceutical non-active ingredientsYolkSide effect
The invention discloses a liposome solid preparation of ozagrel and a preparation method thereof. In the invention, an active ingredient ozagrel hydrochloride and a special combination of hydrogenated yolk lecithin, dioleoyl-phosphatidyl-ethanolamine, cholesteryl succinate, and Span 20 are prepared into a liposome, thereby greatly improving stability, dissolubility, and bioavailability of the drug, and having stable and lasting effect and obvious curative effect. According to the invention, product quality of the preparation is improved, and the toxic side effect is reduced.
Owner:HAINAN YONGTIAN PHARMA INST
A pharmaceutical composition containing ozagrel sodium and its preparation method
ActiveCN106361750BStrong irritantLess irritatingPowder deliveryOrganic active ingredientsNausea sicknessIrritation
Owner:HARBIN ZHENBAO PHARMA +1
Medicinal composition, preparation method and quality control method
InactiveCN1915283AImprove efficiencyImprove bioavailabilityOrganic active ingredientsPharmaceutical delivery mechanismDiseaseCoronary artery disease
A composite medicine in the form of injection or orally taken medicine for treating cardiovascular and cerebrovascular diseases, such as coronary heart disease, angina pectoris, apoplexy sequelae, cerebral thrombus, etc, is prepared from pueraria root or its extract and ozagrel. Its preparing process and quality control method are also disclosed.
Owner:BEIJING QI YUAN YI DE PHARMA RESEARCH CENTER
A kind of preparation method of ozagrel sodium freeze-dried powder injection
ActiveCN105287408BControl contentImprove quality stabilityPowder deliveryOrganic active ingredientsFreeze-dryingRefrigeration
The invention provides a method for preparing a sodium ozagrel freeze-dried powder injection. The method comprises the following steps: a) mixing ozagrel, a sodion-containing alkaline matter and first injection water, and conducting pH value adjustment and adsorption treatment in sequence to obtain a mixed solution A; b) mixing the mixed solution A with second injection water, and conducting refrigeration and drying in sequence so as to obtain the sodium ozagrel freeze-dried powder injection; the volume ratio of the first injection water to the second injection water is 6:(3-5). Compared with the prior art, the provided preparation method can effectively control the content of impurities in the sodium ozagrel freeze-dried powder injection, and can improve the quality stability of the product. Experiment results show that the content of the impurities in the sodium ozagrel freeze-dried powder injection obtained by the provided preparation method is 0.3% or below.
Owner:HUNAN KELUN PHARMA
Pharmaceutical composition with function of treating cerebrovascular diseases
The invention belongs to the technical field of medicine and particularly relates to a pharmaceutical composition with function of treating cerebrovascular diseases. The pharmaceutical composition contains ozagrel sodium and 1, 3-diaza-2, 4-cyclopentadiene, wherein the weight percentage of the ozagrel sodium is more than or equal to 95 percent and less than 100 percent, and the weight percentage of the 1, 3-diaza-2, 4-cyclopentadiene is more than zero and less than or equal to 5 percent. The pharmaceutical composition provided by the invention is determined by pharmacology experiment and toxicology experiment.
Owner:HAINAN BIKAI PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com