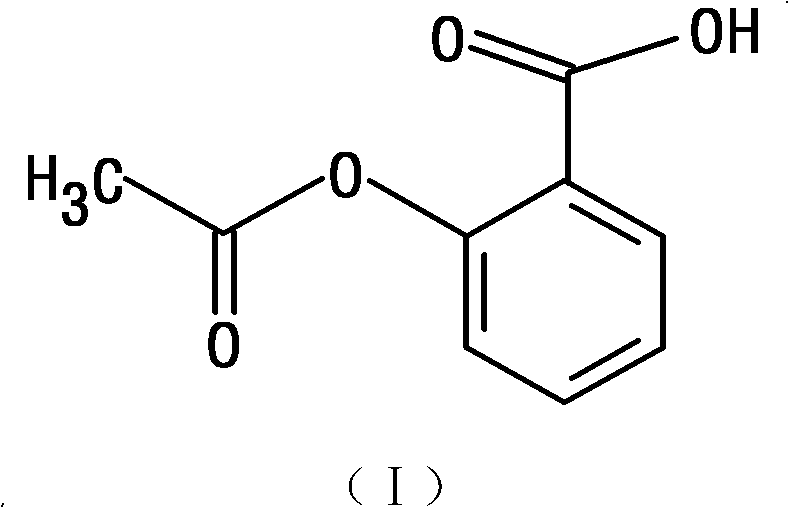
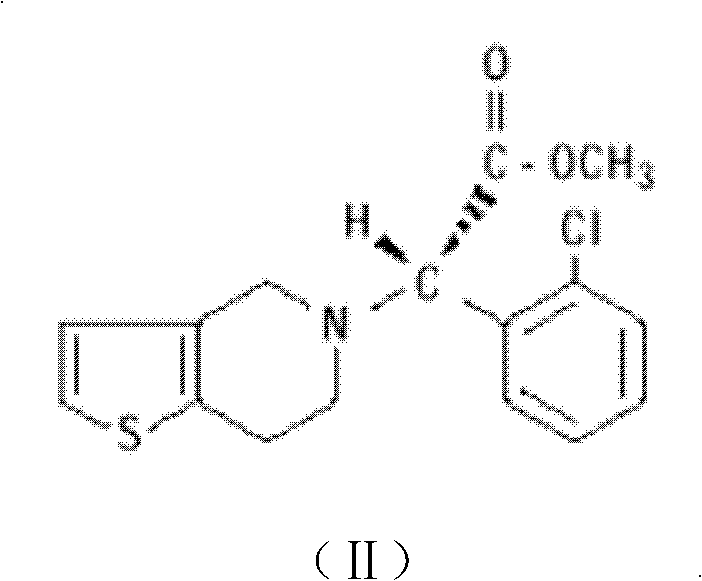
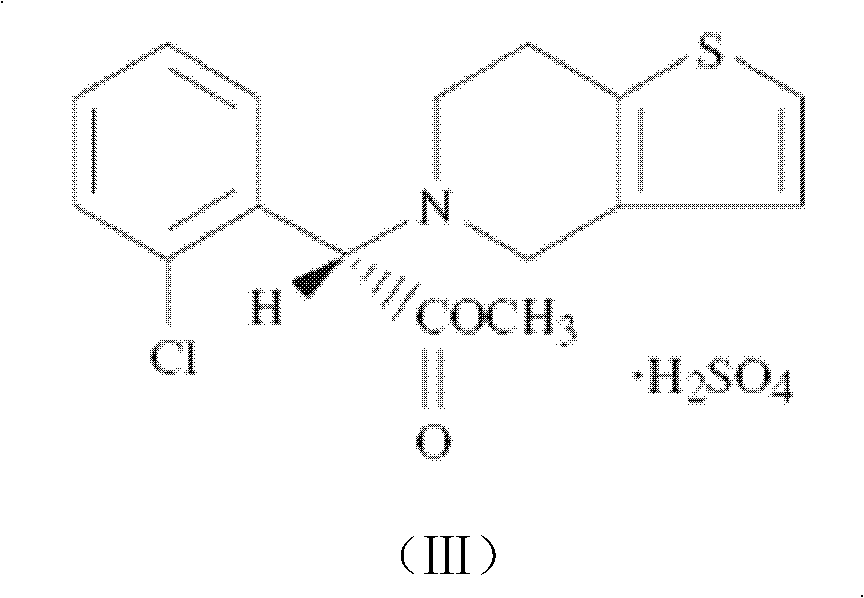
Oral enteric preparation containing Grel drugs and aspirin
A technology for aspirin and enteric-coated preparations, which can be used in medical preparations, drug combinations, and pharmaceutical formulations containing active ingredients, and can solve the problem of high incidence of ulcers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0132] Example 1: Enteric-coated tablet comprising clopidogrel bisulfate and aspirin
[0133] 1.1. Preparation of tablet core: direct compression method
[0134] Name of raw material
Dose 1 (mg)
Dose 2 (mg)
Clopidogrel bisulfate
97.875
97.875
aspirin
75.00
150.00
30.50
30.50
198.215
113.835
Micropowder silica gel
2.05
2.05
60.80
60.80
9.38
18.76
15.00
15.00
hydrogenated castor oil
8.08
8.08
3.10
3.10
Sheet weight
500
500
[0135] Preparation:
[0136] (A) Pass various solid raw and auxiliary materials through 80-100 mesh sieves respectively, and set aside;
[0137] (B) the clopidogrel bisulfate of prescription quantity and micropowder silica gel...
Embodiment 2
[0145] Example 2: Enteric-coated tablet comprising clopidogrel bisulfate and aspirin
[0146] 2.1. Preparation of tablet core: direct compression method
[0147] Name of raw material
[0148] The preparation method of the tablet core is the same as in Example 1, with citric acid replacing tartaric acid.
[0149] 2.2. Preparation of enteric-coated tablets:
[0150] Name of coating excipients
[0152] Preparation method: take hypromellose phthalate and add about 200g of purified water, stir well; add phthalate and talcum powder in the remaining purified water, homogenize in a homogenizer for 45 minutes; suspend the above talcum powder The solution is slowly poured into the aqueous dispersion of hypromellose phthalate, and stirred slowly for 30 minutes; the prepared coating solution is filtered through an 80-mesh screen; 80°C, tablet bed temperature 30-35°C, atomization pressure 1.5bar, coating pan rotation speed 15-25 rpm, sam...
Embodiment 3
[0153] Example 3: Enteric-coated tablet comprising clopidogrel bisulfate and aspirin
[0154] 3.1. Preparation of tablet core: direct compression method
[0155] Name of raw material
[0156] The preparation method of tablet core is the same as embodiment 1.
[0157] 3.2. Preparation of enteric-coated tablets:
[0158] Name of coating excipients
[0159] Preparation method: Take acrylic resin Eudragit L and add about 200g of purified water, stir well; add PEG6000 and talcum powder in the remaining purified water, homogenize in a homogenizer for 45 minutes; slowly pour the above talcum powder suspension into acrylic resin Eudragit L In the water dispersion, stir slowly for 30 minutes; filter the prepared coating solution through an 80-mesh sieve; add 500g of tablet cores to the coating pan, adjust the inlet air temperature to 80°C, the tablet bed temperature to 30-35°C, and atomize The pressure is 1.5 bar, the rotating speed of the coating pan is 15-25 rpm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com