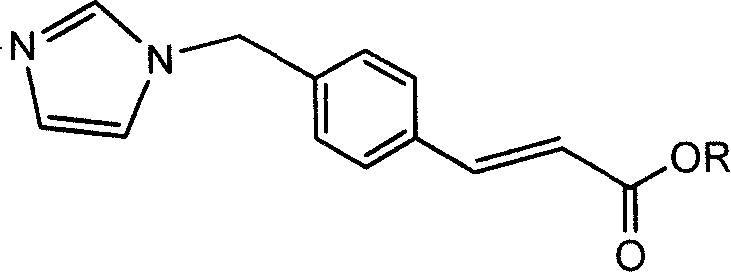
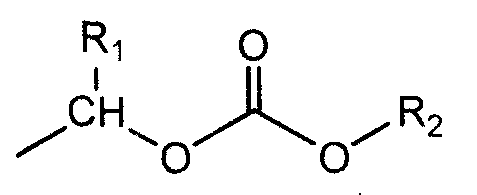
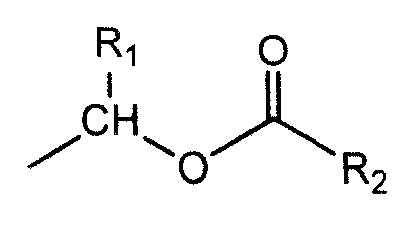
Ozagrel ester, composition and production method thereof
A technology of ozagrel ester and its composition, which is applied in the field of ozagrel ester and its composition and preparation, and can solve the problems of ozagrel ester instability and curative effect reduction, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] These pharmaceutical compositions can be prepared by mixing with suitable pharmaceutical additives such as excipients, disintegrants, binders, lubricants, diluents, buffers or by diluting and dissolving in suitable additives , isotonic agent, preservative, wetting agent, emulsifier, dispersant, stabilizer, solubilizer, etc., and prepare the pharmaceutical composition according to conventional methods.
[0041] When the pharmaceutical composition of the present invention is used for actual treatment, the dose of the compound represented by the above general formula as the active ingredient can be appropriately determined according to the age, sex, body weight, symptoms and degree of treatment of each patient. The dose is approximately 0.1-1,000 mg per adult per day, and the dose is approximately 0.01-500 mg per adult per day for parenteral administration, and the daily dose can be divided into one or several times a day and administered at an appropriate time .
[0042]...
Embodiment 1
[0049] [Example 1] (R, S)-1-(isopropoxycarbonyloxy)-ethyl-(Z)-3[4-(1H-imidazolyl-1-methyl)phenyl]-2- Preparation of Acrylates.
[0050]Put 22.8g of ozagrel into 120ml of DMA, add 2.7g of sodium acetate and 10ml of water under stirring, stir at 10-15°C for 30 minutes, cool to -5°C, add 27.2g of 1-iodoisopropoxycarbonyloxyethane , react at 0-5°C for 2.5 hours, pour into 200ml ethyl acetate, add 100ml 5% sodium bicarbonate solution, stir, separate the organic phase, extract the aqueous phase with 20ml ethyl acetate, combine ethyl acetate, no Dry over magnesium sulfate, filter, and evaporate the solvent under reduced pressure to obtain 52.3 g of white to off-white powder (yield 85%).
[0051] 1 H-NMR (CD 3 Cl, δ) 1.32(d, 6H), 1.76(d, 3H), 4.31(6-fold, 1H), 4.98(s, 2H), 6.40(d, 1H), 6.62(4-fold, 1H), 6.98-7.20(m, 6H), 7.62(d, 1H), 9.26(s, 1H)
Embodiment 2
[0052] [Example 2] Preparation of tert-butylcarbonyloxymethyl-(Z)-3[4-(1H-imidazolyl-1-methyl)phenyl]-2-acrylate.
[0053] Put 22.8g of ozagrel into 120ml of DMA, add 5.5g of sodium isooctanoate and 10ml of water under stirring, stir at 10-15°C for 30 minutes, cool to 0-5°C, add 15.8g of tert-butylcarbonyloxymethane, React at 10-15°C for 2 hours, pour into 200ml of ethyl acetate, then add 100ml of 5% sodium bicarbonate solution, stir, separate the organic phase, extract the aqueous phase with 20ml of ethyl acetate, combine ethyl acetate, anhydrous Dry over magnesium sulfate, filter, and evaporate the solvent under reduced pressure to obtain 27.4 g of white to off-white powder (yield 80%).
[0054] 1 H-NMR (CD 3 Cl, δ) 1.31(s, 9H), 4.96(s, 2H), 6.42(d, 1H), 6.94(s, 2H), 7.15-7.26(m, 6H), 7.68(d, 1H), 9.30(s , 1H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com